Endocytic active zones: hot spots for endocytosis in vertebrate neuromuscular terminals
- PMID: 10366620
- PMCID: PMC6782649
- DOI: 10.1523/JNEUROSCI.19-12-04855.1999
Endocytic active zones: hot spots for endocytosis in vertebrate neuromuscular terminals
Abstract
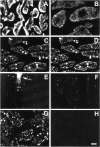
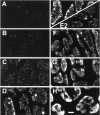
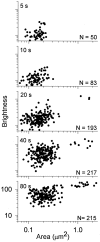
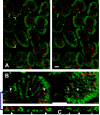
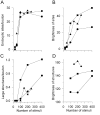
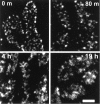
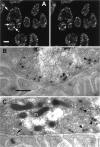
We have used a sensitive activity-dependent probe, sulforhodamine 101 (SR101), to view endocytic events within snake motor nerve terminals. After very brief neural stimulation at reduced temperature, SR101 is visualized exclusively at punctate sites located just inside the presynaptic membrane of each terminal bouton. The number of sites (approximately 26 sites/bouton) and their location (in register with postsynaptic folds) are similar to the number and location of active zones in snake motor terminals, suggesting a spatial association between exocytosis and endocytosis under these stimulus conditions. With more prolonged stimulation, larger SR101-containing structures appear at the bouton margins. Thus endocytosis occurs initially at distinct sites, which we call "endocytic active zones," whereas further stimulation recruits a second endocytic paradigm.
Figures


Similar articles
-
Clathrin-mediated endocytosis near active zones in snake motor boutons.J Neurosci. 2000 Nov 1;20(21):7986-93. doi: 10.1523/JNEUROSCI.20-21-07986.2000. J Neurosci. 2000. PMID: 11050119 Free PMC article.
-
The role of extracellular calcium in exo- and endocytosis of synaptic vesicles at the frog motor nerve terminals.Neuroscience. 2006 Dec 28;143(4):905-10. doi: 10.1016/j.neuroscience.2006.08.025. Epub 2006 Sep 26. Neuroscience. 2006. PMID: 17000054
-
Macroendocytosis and endosome processing in snake motor boutons.J Physiol. 2007 Jul 1;582(Pt 1):243-62. doi: 10.1113/jphysiol.2007.130989. Epub 2007 May 3. J Physiol. 2007. PMID: 17478535 Free PMC article.
-
Exocytosis and endocytosis of synaptic vesicles and functional roles of vesicle pools: lessons from the Drosophila neuromuscular junction.Neuroscientist. 2005 Apr;11(2):138-47. doi: 10.1177/1073858404271679. Neuroscientist. 2005. PMID: 15746382 Review.
-
Restoring synaptic vesicles during compensatory endocytosis.Essays Biochem. 2015;57:121-34. doi: 10.1042/bse0570121. Essays Biochem. 2015. PMID: 25658349 Review.
Cited by
-
Interaction of stoned and synaptotagmin in synaptic vesicle endocytosis.J Neurosci. 2001 Feb 15;21(4):1218-27. doi: 10.1523/JNEUROSCI.21-04-01218.2001. J Neurosci. 2001. PMID: 11160392 Free PMC article.
-
Bulk membrane retrieval in the synaptic terminal of retinal bipolar cells.J Neurosci. 2003 Feb 15;23(4):1329-39. doi: 10.1523/JNEUROSCI.23-04-01329.2003. J Neurosci. 2003. PMID: 12598621 Free PMC article.
-
Heterogeneity in synaptic vesicle release at neuromuscular synapses of mice expressing synaptopHluorin.J Neurosci. 2008 Jan 2;28(1):325-35. doi: 10.1523/JNEUROSCI.3544-07.2008. J Neurosci. 2008. PMID: 18171949 Free PMC article.
-
Clathrin-independent internalization and recycling.J Cell Mol Med. 2008 Jan-Feb;12(1):126-44. doi: 10.1111/j.1582-4934.2007.00148.x. Epub 2007 Nov 26. J Cell Mol Med. 2008. PMID: 18039352 Free PMC article. Review.
-
Disruption of actin impedes transmitter release in snake motor terminals.J Physiol. 2000 Jun 15;525 Pt 3(Pt 3):579-86. doi: 10.1111/j.1469-7793.2000.t01-2-00579.x. J Physiol. 2000. PMID: 10856113 Free PMC article.
References
-
- Anderson RGW, Brown MS, Goldstein JL. Role of the coated endocytic vesicle in the uptake of receptor-bound low density lipoprotein in human fibroblasts. Cell. 1977;10:351–364. - PubMed
-
- Angleson JK, Betz WJ. Monitoring secretion in real time: capacitance, amperometry and fluorescence compared. Trends Neurosci. 1997;20:281–287. - PubMed
-
- Balice-Gordon RJ, Chua CK, Nelson CC, Lichtman JW. Gradual loss of synaptic cartels precedes axon withdrawal at developing neuromuscular junctions. Neuron. 1993;11:801–815. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
