Netrin-3, a mouse homolog of human NTN2L, is highly expressed in sensory ganglia and shows differential binding to netrin receptors
- PMID: 10366627
- PMCID: PMC6782680
- DOI: 10.1523/JNEUROSCI.19-12-04938.1999
Netrin-3, a mouse homolog of human NTN2L, is highly expressed in sensory ganglia and shows differential binding to netrin receptors
Abstract
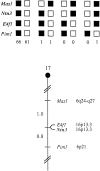
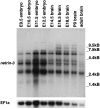
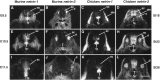
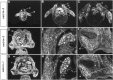
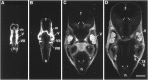
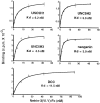
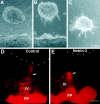
The netrins comprise a small phylogenetically conserved family of guidance cues important for guiding particular axonal growth cones to their targets. Two netrin genes, netrin-1 and netrin-2, have been described in chicken, but in mouse so far a single netrin gene, an ortholog of chick netrin-1, has been reported. We report the identification of a second mouse netrin gene, which we name netrin-3. Netrin-3 does not appear to be the ortholog of chick netrin-2 but is the ortholog of a recently identified human netrin gene termed NTN2L ("netrin-2-like"), as evidenced by a high degree of sequence conservation and by chromosomal localization. Netrin-3 is expressed in sensory ganglia, mesenchymal cells, and muscles during the time of peripheral nerve development but is largely excluded from the CNS at early stages of its development. The murine netrin-3 protein binds to netrin receptors of the DCC (deleted in colorectal cancer) family [DCC and neogenin] and the UNC5 family (UNC5H1, UNC5H2 and UNC5H3). Unlike chick netrin-1, however, murine netrin-3 binds to DCC with lower affinity than to the other four receptors. Consistent with this finding, although murine netrin-3 can mimic the outgrowth-promoting activity of netrin-1 on commissural axons, it has lower specific activity than netrin-1. Thus, like netrin-1, netrin-3 may also function in axon guidance during development but may function predominantly in the development of the peripheral nervous system and may act primarily through netrin receptors other than DCC.
Figures


Similar articles
-
The netrin protein family.Genome Biol. 2009;10(9):239. doi: 10.1186/gb-2009-10-9-239. Epub 2009 Sep 29. Genome Biol. 2009. PMID: 19785719 Free PMC article. Review.
-
Netrin 1 and Dcc signalling are required for confinement of central axons within the central nervous system.Development. 2014 Feb;141(3):594-603. doi: 10.1242/dev.099606. Development. 2014. PMID: 24449837
-
Divergent properties of mouse netrins.Mech Dev. 1999 May;83(1-2):65-75. doi: 10.1016/s0925-4773(99)00035-0. Mech Dev. 1999. PMID: 10381568
-
Genetic analysis of Netrin genes in Drosophila: Netrins guide CNS commissural axons and peripheral motor axons.Neuron. 1996 Aug;17(2):203-15. doi: 10.1016/s0896-6273(00)80153-1. Neuron. 1996. PMID: 8780645
-
Netrins and their receptors.Adv Exp Med Biol. 2007;621:17-31. doi: 10.1007/978-0-387-76715-4_2. Adv Exp Med Biol. 2007. PMID: 18269208 Review.
Cited by
-
Netrin-G1: a novel glycosyl phosphatidylinositol-linked mammalian netrin that is functionally divergent from classical netrins.J Neurosci. 2000 Sep 1;20(17):6540-50. doi: 10.1523/JNEUROSCI.20-17-06540.2000. J Neurosci. 2000. PMID: 10964959 Free PMC article.
-
Axon guidance by diffusible chemoattractants: a gradient of netrin protein in the developing spinal cord.J Neurosci. 2006 Aug 23;26(34):8866-74. doi: 10.1523/JNEUROSCI.5191-05.2006. J Neurosci. 2006. PMID: 16928876 Free PMC article.
-
The netrin protein family.Genome Biol. 2009;10(9):239. doi: 10.1186/gb-2009-10-9-239. Epub 2009 Sep 29. Genome Biol. 2009. PMID: 19785719 Free PMC article. Review.
-
Molecular/genetic manipulation of extrinsic axon guidance factors for CNS repair and regeneration.Exp Neurol. 2008 Feb;209(2):333-42. doi: 10.1016/j.expneurol.2007.06.026. Epub 2007 Jul 21. Exp Neurol. 2008. PMID: 17706643 Free PMC article. Review.
-
A Specialized Reference Panel with Structural Variants Integration for Improving Genotype Imputation in Alzheimer's Disease and Related Dementias (ADRD).medRxiv [Preprint]. 2024 Jul 23:2024.07.22.24310827. doi: 10.1101/2024.07.22.24310827. medRxiv. 2024. Update in: HGG Adv. 2025 Jul 31;6(4):100487. doi: 10.1016/j.xhgg.2025.100487. PMID: 39108532 Free PMC article. Updated. Preprint.
References
-
- Ackerman SL, Kozak LP, Przyborski SA, Rund LA, Boyer BB, Knowles BB. The mouse rostral cerebellar malformation gene encodes an UNC-5-like protein. Nature. 1997;386:838–842. - PubMed
-
- Chan SS, Zheng H, Su MW, Wilk R, Killeen MT, Hedgecock EM, Culotti JG. UNC-40, a C. elegans homolog of DCC (deleted in colorectal cancer), is required in motile cells responding to UNC-6 netrin cues. Cell. 1996;87:187–195. - PubMed
-
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenochloroform extraction. Anal Biochem. 1987;162:156–159. - PubMed
-
- Colamarino SA, Tessier-Lavigne M. The axonal chemoattractant netrin-1 is also a chemorepellent for trochlear motor axons. Cell. 1995;81:621–629. - PubMed
-
- Copeland NG, Jenkins N. Development and applications of a molecular genetic linkage map of the mouse genome. Trends Genet. 1991;7:113–118. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
