Positionally selective growth of embryonic spinal cord neurites on muscle membranes
- PMID: 10366631
- PMCID: PMC6782665
- DOI: 10.1523/JNEUROSCI.19-12-04984.1999
Positionally selective growth of embryonic spinal cord neurites on muscle membranes
Abstract
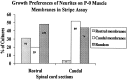
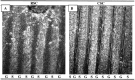
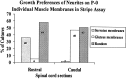
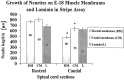
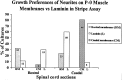
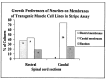
Motor neurons from distinct positions along the rostrocaudal axis generally innervate muscles or muscle fibers from corresponding axial levels. These topographic maps of connectivity are partially restored after denervation or transplantation under conditions in which factors of timing and proximity are eliminated. It is therefore likely that motor neurons and some intramuscular structures bear cues that bias synapse formation in favor of positionally matched partners. To localize these cues, we studied outgrowth of neurites from embryonic spinal cord explants on carpets of membranes isolated from perinatal rat muscles. Neurites from rostral (cervical) and caudal (lumbar) spinal cord slices exhibit distinct growth preferences. In many instances, rostrally derived neurites grew selectively on membranes from forelimb muscles or from a single thoracic muscle (the serratus anterior) when given a choice between these membranes and membranes from hindlimb muscles or laminin. Caudally derived neurites almost never exhibited such rostral preferences, but instead preferred membranes from hindlimb muscles or a single hindlimb muscle (the gluteus) to rostral muscles or laminin. Likewise, spinal neurites exhibited distinct position-related preferences for outgrowth on membranes of clonal myogenic cell lines derived from specific rostral and caudal muscles. Taken together these results suggest that the membranes of motor axons and myotubes bear complementary labels that vary with rostrocaudal position and regulate neuromuscular connectivity.
Figures




Similar articles
-
Topographic specificity within membranes of a single muscle detected in vitro.J Neurosci. 2007 Dec 19;27(51):13938-48. doi: 10.1523/JNEUROSCI.3055-07.2007. J Neurosci. 2007. PMID: 18094231 Free PMC article.
-
Development of inhibition by ephrin-A5 on outgrowth of embryonic spinal motor neurites.J Neurobiol. 2001 Jun 5;47(3):233-43. doi: 10.1002/neu.1030. J Neurobiol. 2001. PMID: 11333404
-
Roles for ephrins in positionally selective synaptogenesis between motor neurons and muscle fibers.Neuron. 2000 Feb;25(2):295-306. doi: 10.1016/s0896-6273(00)80895-8. Neuron. 2000. PMID: 10719886
-
The rostrocaudal organization in the dorsal root ganglia of the rat: a consequence of plexus formation?Anat Embryol (Berl). 1994 Jul;190(1):1-11. doi: 10.1007/BF00185841. Anat Embryol (Berl). 1994. PMID: 7985809 Review.
-
Patterning of skeletal muscle.Curr Opin Neurobiol. 2002 Feb;12(1):100-3. doi: 10.1016/s0959-4388(02)00296-9. Curr Opin Neurobiol. 2002. PMID: 11861171 Review.
Cited by
-
Topographic specificity within membranes of a single muscle detected in vitro.J Neurosci. 2007 Dec 19;27(51):13938-48. doi: 10.1523/JNEUROSCI.3055-07.2007. J Neurosci. 2007. PMID: 18094231 Free PMC article.
-
Motor axon pathfinding.Cold Spring Harb Perspect Biol. 2010 Mar;2(3):a001735. doi: 10.1101/cshperspect.a001735. Cold Spring Harb Perspect Biol. 2010. PMID: 20300210 Free PMC article. Review.
References
-
- Bennett MR, Lavidis NA. Segmental motor projections to rat muscles during the loss of polyneuronal innervation. Dev Brain Res. 1984a;13:1–7. - PubMed
-
- Brown MC, Booth CM. Segregation of motor nerves on a segmental basis during synapse elimination in neonatal muscles. Brain Res. 1983a;273:188–190. - PubMed
-
- Brown MC, Booth CM. Postnatal development of the adult pattern of motor axon distribution in rat muscles. Nature. 1983b;304:741–742. - PubMed
-
- Brown MC, Hardman VJ. A reassessment of the accuracy of reinnervation by motoneurons following crushing or freezing of the sciatic or lumbar spinal nerves of rats. Brain. 1987;110:695–705. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
