Modulation of learning and anxiety by corticotropin-releasing factor (CRF) and stress: differential roles of CRF receptors 1 and 2
- PMID: 10366634
- PMCID: PMC6782638
- DOI: 10.1523/JNEUROSCI.19-12-05016.1999
Modulation of learning and anxiety by corticotropin-releasing factor (CRF) and stress: differential roles of CRF receptors 1 and 2
Abstract
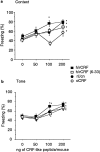
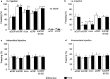
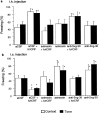
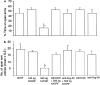
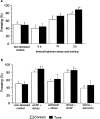
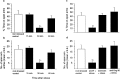
The differential modulation of learning and anxiety by corticotropin-releasing factor (CRF) through CRF receptor subtypes 1 (CRFR1) and 2 (CRFR2) is demonstrated. As learning paradigm, context- and tone-dependent fear conditioning of the mouse was used. Injection of CRF into the dorsal hippocampus before training enhanced learning through CRFR1 as demonstrated by the finding that this effect was prevented by the local injection of the unselective CRFR antagonist astressin, but not by the CRFR2-specific antagonist antisauvagine-30 (anti-Svg-30). In contrast, injection of CRF into the lateral intermediate septum impaired learning through CRFR2, as demonstrated by the ability of antisauvagine-30 to block this effect. When antisauvagine-30 was injected alone into the lateral intermediate septum, learning was enhanced. Such tonic control of learning was not observed when astressin or antisauvagine-30 was injected into the dorsal hippocampus. Injection of CRF after the training into the dorsal hippocampus and the lateral intermediate septum also enhanced and impaired learning, respectively. Thus, it was indicated that CRF acted on memory consolidation. It was concluded that the observed effects reflected changes of associative learning and not arousal, attention, or motivation. Although a dose of 20 pmol human/rat CRF was sufficient to affect learning significantly, a fivefold higher dose was required to induce anxiety by injection into the septum. Immobilization for 1 hr generated a stress response that included the induction of anxiety through septal CRFR2 and the subsequent enhancement of learning through hippocampal CRFR1. The involvement of either receptor subtype was demonstrated by region-specific injections of astressin and antisauvagine-30.
Figures



Similar articles
-
Differential activation of CRF receptor subtypes removes stress-induced memory deficit and anxiety.Eur J Neurosci. 2007 Jun;25(11):3385-97. doi: 10.1111/j.1460-9568.2007.05592.x. Eur J Neurosci. 2007. PMID: 17553007
-
Antagonism of CRF(2) receptors produces anxiolytic behavior in animal models of anxiety.Brain Res. 2001 Jun 1;902(2):135-42. doi: 10.1016/s0006-8993(01)02405-2. Brain Res. 2001. PMID: 11384606
-
The blockage of ventromedial hypothalamus CRF type 2 receptors impairs escape responses in the elevated T-maze.Behav Brain Res. 2017 Jun 30;329:41-50. doi: 10.1016/j.bbr.2017.04.030. Epub 2017 Apr 21. Behav Brain Res. 2017. PMID: 28435125
-
Actions of CRF and its analogs.Curr Med Chem. 1999 Nov;6(11):1035-53. Curr Med Chem. 1999. PMID: 10519912 Review.
-
Corticotropin-releasing factor peptide antagonists: design, characterization and potential clinical relevance.Front Neuroendocrinol. 2014 Apr;35(2):161-70. doi: 10.1016/j.yfrne.2013.10.006. Epub 2013 Nov 20. Front Neuroendocrinol. 2014. PMID: 24269930 Free PMC article. Review.
Cited by
-
Postpubertal sex differentiation of forebrain structures and functions depend on transforming growth factor-alpha.J Neurosci. 2005 Apr 13;25(15):3870-80. doi: 10.1523/JNEUROSCI.0175-05.2005. J Neurosci. 2005. PMID: 15829639 Free PMC article.
-
Divergent effects of corticotropin releasing hormone on endothelial cell nitric oxide synthase are associated with different expression of CRH type 1 and 2 receptors.Br J Pharmacol. 2001 Oct;134(4):837-44. doi: 10.1038/sj.bjp.0704322. Br J Pharmacol. 2001. PMID: 11606324 Free PMC article.
-
Stress and memory: behavioral effects and neurobiological mechanisms.Neural Plast. 2007;2007:78970. doi: 10.1155/2007/78970. Neural Plast. 2007. PMID: 18060012 Free PMC article. Review.
-
Corticotropin-releasing factor receptors couple to multiple G-proteins to activate diverse intracellular signaling pathways in mouse hippocampus: role in neuronal excitability and associative learning.J Neurosci. 2003 Jan 15;23(2):700-7. doi: 10.1523/JNEUROSCI.23-02-00700.2003. J Neurosci. 2003. PMID: 12533630 Free PMC article.
-
Protein profiles associated with context fear conditioning and their modulation by memantine.Mol Cell Proteomics. 2014 Apr;13(4):919-37. doi: 10.1074/mcp.M113.035568. Epub 2014 Jan 27. Mol Cell Proteomics. 2014. PMID: 24469516 Free PMC article.
References
-
- Aiba A, Chen C, Herrup K, Rosenmund C, Stevens C F, Tonegawa Reduced hippocampal long-term potentiation and context-specific deficit in associative learning in mGluR1 mutant mice. Cell. 1994;79:366–375. - PubMed
-
- Behan DP, Heinrichs SC, Troncoso JC, Liu XJ, Kawas CH, Ling N, De Souza EB. Displacement of corticotropin releasing factor from its binding protein as a possible treatment for Alzheimer’s disease. Nature. 1995;378:284–287. - PubMed
-
- Behan D, Khongsaly O, Ling N, De Souza EB. Urocortin interaction with corticotropin-releasing factor (CRF) binding protein (CRF-BP): a novel mechanism for elevating “free” CRF levels in human brain. Brain Res. 1996;725:263–267. - PubMed
-
- Cador M, Ahmed S H, Koob GF, Le Moal M, Stinus L. Corticotropin-releasing factor induces a place aversion independent of its neuroendocrine role. Brain Res. 1992;597:304–309. - PubMed
-
- Chang CP, Pearse RV, O’Connell S, Rosenfeld MG. Identification of a seven transmembrane helix receptor for corticotropin-releasing factor and sauvagine in mammalian brain. Neuron. 1993;11:1187–1195. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
