OCD-Like behaviors caused by a neuropotentiating transgene targeted to cortical and limbic D1+ neurons
- PMID: 10366637
- PMCID: PMC6782675
- DOI: 10.1523/JNEUROSCI.19-12-05044.1999
OCD-Like behaviors caused by a neuropotentiating transgene targeted to cortical and limbic D1+ neurons
Abstract
To study the behavioral role of neurons containing the D1 dopamine receptor (D1+), we have used a genetic neurostimulatory approach. We generated transgenic mice that express an intracellular form of cholera toxin (CT), a neuropotentiating enzyme that chronically activates stimulatory G-protein (Gs) signal transduction and cAMP synthesis, under the control of the D1 promoter. Because the D1 promoter, like other CNS-expressed promoters, confers transgene expression that is regionally restricted to different D1+ CNS subsets in different transgenic lines, we observed distinct but related psychomotor disorders in different D1CT-expressing founders. In a D1CT line in which transgene expression was restricted to the following D1+ CNS regions-the piriform cortex layer II, layers II-III of somatosensory cortical areas, and the intercalated nucleus of the amygdala-D1CT mice showed normal CNS and D1+ neural architecture but increased cAMP content in whole extracts of the piriform and somatosensory cortex. These mice also exhibited a constellation of compulsive behavioral abnormalities that strongly resembled human cortical-limbic-induced compulsive disorders such as obsessive-compulsive disorder (OCD). These compulsive behaviors included episodes of perseverance or repetition of any and all normal behaviors, repetitive nonaggressive biting of siblings during grooming, and repetitive leaping. These results suggest that chronic potentiation of cortical and limbic D1+ neurons thought to induce glutamatergic output to the striatum causes behaviors reminiscent of those in human cortical-limbic-induced compulsive disorders.
Figures

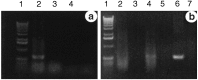
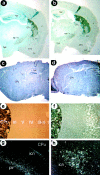



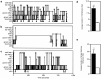
Similar articles
-
Behavioral effects of cocaine on a transgenic mouse model of cortical-limbic compulsion.Brain Res. 1999 Jul 3;833(2):216-24. doi: 10.1016/s0006-8993(99)01544-9. Brain Res. 1999. PMID: 10375697
-
Anxiety in a transgenic mouse model of cortical-limbic neuro-potentiated compulsive behavior.Behav Pharmacol. 1999 Sep;10(5):435-43. doi: 10.1097/00008877-199909000-00001. Behav Pharmacol. 1999. PMID: 10780249
-
Differential response of cortical-limbic neuropotentiated compulsive mice to dopamine D1 and D2 receptor antagonists.Eur J Pharmacol. 1999 Apr 29;371(2-3):103-11. doi: 10.1016/s0014-2999(99)00184-3. Eur J Pharmacol. 1999. PMID: 10357247
-
Peromyscus maniculatus bairdii as a naturalistic mammalian model of obsessive-compulsive disorder: current status and future challenges.Metab Brain Dis. 2018 Apr;33(2):443-455. doi: 10.1007/s11011-017-0161-7. Epub 2017 Dec 6. Metab Brain Dis. 2018. PMID: 29214602 Review.
-
The role of central oxytocin in obsessive compulsive disorder and related normal behavior.Psychoneuroendocrinology. 1994;19(8):723-49. doi: 10.1016/0306-4530(94)90021-3. Psychoneuroendocrinology. 1994. PMID: 7991761 Review.
Cited by
-
DISSECTING OCD CIRCUITS: FROM ANIMAL MODELS TO TARGETED TREATMENTS.Depress Anxiety. 2015 Aug;32(8):550-62. doi: 10.1002/da.22367. Epub 2015 May 7. Depress Anxiety. 2015. PMID: 25952989 Free PMC article. Review.
-
Norepinephrine and dopamine contribute to distinct repetitive behaviors induced by novel odorant stress in male and female mice.Horm Behav. 2022 Aug;144:105205. doi: 10.1016/j.yhbeh.2022.105205. Epub 2022 Jun 1. Horm Behav. 2022. PMID: 35660247 Free PMC article.
-
Animal models of OCD-relevant processes: an RDoC perspective.J Obsessive Compuls Relat Disord. 2019 Oct;23:100433. doi: 10.1016/j.jocrd.2019.03.001. Epub 2019 Apr 3. J Obsessive Compuls Relat Disord. 2019. PMID: 32322462 Free PMC article.
-
Reverse-translational biomarker validation of Abnormal Repetitive Behaviors in mice: an illustration of the 4P's modeling approach.Behav Brain Res. 2011 Jun 1;219(2):189-96. doi: 10.1016/j.bbr.2011.01.002. Epub 2011 Jan 8. Behav Brain Res. 2011. PMID: 21219937 Free PMC article.
-
Animal models of obsessive-compulsive disorder: utility and limitations.Neuropsychiatr Dis Treat. 2015 Aug 4;11:1939-55. doi: 10.2147/NDT.S62785. eCollection 2015. Neuropsychiatr Dis Treat. 2015. PMID: 26346234 Free PMC article. Review.
References
-
- Abel T, Nguyen PV, Borad M, Deuel TA, Kandel ER, Bourtchouladze R. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell. 1997;88:615–626. - PubMed
-
- Alleva E. Assessment of aggressive behavior in rodents. Methods Neurosci. 1993;14:111–137.
-
- Ariano MA, Sibley DR. Dopamine receptor distribution in the rat CNS: elucidation using anti-peptide antisera directed against D1A and D3 subtypes. Brain Res. 1994;649:95–110. - PubMed
-
- Bach ME, Hawkins RD, Osman M, Kandel ER, Mayford M. Impairment of spatial but not contextual memory in CaMKII mutant mice with a selective loss of hippocampal LTP in the range of the theta frequency. Cell. 1995;81:905–915. - PubMed
-
- Bachus SE, Kleinman JE. The neuropathology of schizophrenia. J Clin Psychiatry. 1996;57:72–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
