A Bacillus subtilis secreted protein with a role in endospore coat assembly and function
- PMID: 10368135
- PMCID: PMC93838
- DOI: 10.1128/JB.181.12.3632-3643.1999
A Bacillus subtilis secreted protein with a role in endospore coat assembly and function
Abstract
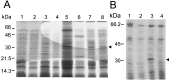
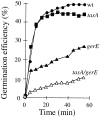
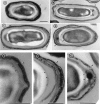
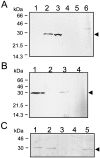
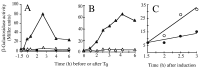
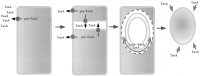
Bacterial endospores are encased in a complex protein coat, which confers protection against noxious chemicals and influences the germination response. In Bacillus subtilis, over 20 polypeptides are organized into an amorphous undercoat, a lamellar lightly staining inner structure, and an electron-dense outer coat. Here we report on the identification of a polypeptide of about 30 kDa required for proper coat assembly, which was extracted from spores of a gerE mutant. The N-terminal sequence of this polypeptide matched the deduced product of the tasA gene, after removal of a putative 27-residue signal peptide, and TasA was immunologically detected in material extracted from purified spores. Remarkably, deletion of tasA results in the production of asymmetric spores that accumulate misassembled material in one pole and have a greatly expanded undercoat and an altered outer coat structure. Moreover, we found that tasA and gerE mutations act synergistically to decrease the efficiency of spore germination. We show that tasA is the most distal member of a three-gene operon, which also encodes the type I signal peptidase SipW. Expression of the tasA operon is enhanced 2 h after the onset of sporulation, under the control of sigmaH. When tasA transcription is uncoupled from sipW expression, a presumptive TasA precursor accumulates, suggesting that its maturation depends on SipW. Mature TasA is found in supernatants of sporulating cultures and intracellularly from 2 h of sporulation onward. We suggest that, at an early stage of sporulation, TasA is secreted to the septal compartment. Later, after engulfment of the prespore by the mother cell, TasA acts from the septal-proximal pole of the spore membranes to nucleate the organization of the undercoat region. TasA is the first example of a polypeptide involved in coat assembly whose production is not mother cell specific but rather precedes its formation. Our results implicate secretion as a mechanism to target individual proteins to specific cellular locations during the assembly of the bacterial endospore coat.
Figures

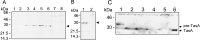
Similar articles
-
Characterization of cotJ, a sigma E-controlled operon affecting the polypeptide composition of the coat of Bacillus subtilis spores.J Bacteriol. 1995 Jun;177(12):3394-406. doi: 10.1128/jb.177.12.3394-3406.1995. J Bacteriol. 1995. PMID: 7768848 Free PMC article.
-
Secretion, localization, and antibacterial activity of TasA, a Bacillus subtilis spore-associated protein.J Bacteriol. 1999 Mar;181(5):1664-72. doi: 10.1128/JB.181.5.1664-1672.1999. J Bacteriol. 1999. PMID: 10049401 Free PMC article.
-
Cloning and characterization of a gene required for assembly of the Bacillus subtilis spore coat.J Bacteriol. 1993 Mar;175(6):1705-16. doi: 10.1128/jb.175.6.1705-1716.1993. J Bacteriol. 1993. PMID: 8449878 Free PMC article.
-
Structure and assembly of the bacterial endospore coat.Methods. 2000 Jan;20(1):95-110. doi: 10.1006/meth.1999.0909. Methods. 2000. PMID: 10610808 Review.
-
Maximum shields: the assembly and function of the bacterial spore coat.Trends Microbiol. 2002 Jun;10(6):251-4. doi: 10.1016/s0966-842x(02)02373-9. Trends Microbiol. 2002. PMID: 12088650 Review.
Cited by
-
Biofilm research uncovers a novel nonenzymatic signal peptidase function in Bacillus.J Bacteriol. 2012 Jun;194(11):2779-80. doi: 10.1128/JB.00270-12. Epub 2012 Mar 16. J Bacteriol. 2012. PMID: 22427624 Free PMC article. No abstract available.
-
Signal peptide-dependent protein transport in Bacillus subtilis: a genome-based survey of the secretome.Microbiol Mol Biol Rev. 2000 Sep;64(3):515-47. doi: 10.1128/MMBR.64.3.515-547.2000. Microbiol Mol Biol Rev. 2000. PMID: 10974125 Free PMC article. Review.
-
Transcriptome and proteome analysis of Bacillus subtilis gene expression modulated by amino acid availability.J Bacteriol. 2002 Aug;184(15):4288-95. doi: 10.1128/JB.184.15.4288-4295.2002. J Bacteriol. 2002. PMID: 12107147 Free PMC article.
-
Expression of spoIIIJ in the prespore is sufficient for activation of sigma G and for sporulation in Bacillus subtilis.J Bacteriol. 2003 Jul;185(13):3905-17. doi: 10.1128/JB.185.13.3905-3917.2003. J Bacteriol. 2003. PMID: 12813085 Free PMC article.
-
Genes involved in formation of structured multicellular communities by Bacillus subtilis.J Bacteriol. 2004 Jun;186(12):3970-9. doi: 10.1128/JB.186.12.3970-3979.2004. J Bacteriol. 2004. PMID: 15175311 Free PMC article.
References
-
- Aronson A I, Song H-Y, Bourne N. Gene structure and precursor processing of a novel Bacillus subtilisspore coat protein. Mol Microbiol. 1988;3:437–444. - PubMed
-
- Aronson A I, Ekanayake L, Fitz-James P C. Protein filaments may initiate the assembly of the Bacillus subtilisspore coat. Biochimie. 1992;74:661–667. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

