Characterization of the vanD glycopeptide resistance gene cluster from Enterococcus faecium BM4339
- PMID: 10368136
- PMCID: PMC93839
- DOI: 10.1128/JB.181.12.3644-3648.1999
Characterization of the vanD glycopeptide resistance gene cluster from Enterococcus faecium BM4339
Abstract
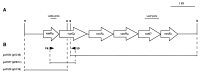
VanD-type resistance to glycopeptides in Enterococcus faecium BM4339 is due to constitutive synthesis of D-alanyl-D-lactate-terminating peptidoglycan precursors (B. Périchon, P. Reynolds, and P. Courvalin, Antimicrob. Agents Chemother. 41:2016-2018, 1997). The sequence of a 5,780-bp fragment was determined and revealed six open reading frames. The 3' distal part encoded the VanHD dehydrogenase, the VanD ligase, and the VanXD DD-dipeptidase, which were highly similar to the corresponding proteins in VanA and VanB types of resistance. The deduced VanYD protein was homologous to penicillin-binding proteins that display DD-carboxypeptidase activity. The 5' end coded for the putative VanRD-VanSD two-component regulatory system. Due to a frameshift mutation in the chromosomal ddl gene, BM4339 produced an impaired D-alanine:D-alanine ligase. However, since expression of the resistance genes is constitutive, growth of E. faecium BM4339 was not dependent on the presence of glycopeptides in the culture medium.
Figures
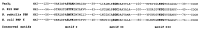

References
-
- Arthur, M., et al. Unpublished data.
-
- Arthur M, Depardieu F, Cabanié L, Reynolds P, Courvalin P. Requirement of the VanY and VanX d,d-peptidases for glycopeptide resistance in enterococci. Mol Microbiol. 1998;31:819–830. - PubMed
-
- Arthur M, Molinas C, Courvalin P. Sequence of the vanY gene required for production of a vancomycin-inducible d,d-carboxypeptidase in Enterococcus faeciumBM4147. Gene. 1992;120:111–114. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

