Effects of (-)-tertatolol, (-)-penbutolol and (+/-)-pindolol in combination with paroxetine on presynaptic 5-HT function: an in vivo microdialysis and electrophysiological study
- PMID: 10369467
- PMCID: PMC1566011
- DOI: 10.1038/sj.bjp.0702546
Effects of (-)-tertatolol, (-)-penbutolol and (+/-)-pindolol in combination with paroxetine on presynaptic 5-HT function: an in vivo microdialysis and electrophysiological study
Abstract
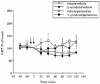
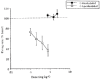
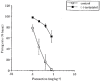
The antidepressant efficacy of selective serotonin reuptake inhibitors (SSRIs) might be enhanced by co-administration of 5-HT1A receptor antagonists. Thus, we have recently shown that the selective 5-HT1A receptor antagonist, WAY 100635, blocks the inhibitory effect of an SSRI on 5-HT cell firing, and enhances its ability to elevate extracellular 5-HT in the forebrain. Here we determined whether the beta-adrenoceptor/5-HT1A receptor ligands (+/-)-pindolol, (-)-tertatolol and (-)-penbutolol, interact with paroxetine in a similar manner. Both (-)-tertatolol (2.4 mg kg(-1) i.v.) and (-)-penbutolol (2.4 mg kg(-1) i.v.) enhanced the effect of paroxetine (0.8 mg kg(-1) i.v.) on extracellular 5-HT in the frontal cortex, whilst (+/-)-pindolol (4 mg kg(-1) i.v.) did not. (-)-Tertatolol (2.4 mg kg(-1) i.v.) alone caused a slight increase in 5-HT however, (-)-penbutolol (2.4 mg kg(-1) i.v.) alone had no effect. In electrophysiological studies (-)-tertatolol (2.4 mg kg(-1) i.v.) alone had no effect on 5-HT cell firing but blocked the inhibitory effect of paroxetine. In contrast, (-)-penbutolol (0.1-0.8 mg kg(-1) i.v.) itself inhibited 5-HT cell firing, and this effect was reversed by WAY 100635 (0.1 mg kg(-1) i.v.). We have recently shown that (+/-)-pindolol inhibits 5-HT cell firing via a WAY 100635-sensitive mechanism. Our data suggest that (-)-tertatolol enhances the effect of paroxetine on forebrain 5-HT via blockade of 5-HT1A autoreceptors which mediate paroxetine-induced inhibition of 5-HT cell firing. In comparison, the mechanisms by which (-)-penbutolol enhances the effect of paroxetine on extracellular 5-HT is unclear, since (-)-penbutolol itself appears to have agonist properties at the 5-HT1A autoreceptor. Indeed, the agonist action of (+/-)-pindolol at 5-HT1A autoreceptors probably explains its inability to enhance the effect of paroxetine on 5-HT in the frontal cortex. Overall, our data suggest that both (-)-tertatolol and (-)-penbutolol are superior to (+/-)-pindolol in terms of enhancing the effect of an SSRI on extracellular 5-HT. Both (-)-tertatolol and (-)-penbutolol are worthy of investigation for use as adjuncts to SSRIs in the treatment of major depression.
Figures








Similar articles
-
Affinity of (+/-)-pindolol, (-)-penbutolol, and (-)-tertatolol for pre- and postsynaptic serotonin 5-HT(1A) receptors in human and rat brain.J Neurochem. 2000 Aug;75(2):755-62. doi: 10.1046/j.1471-4159.2000.0750755.x. J Neurochem. 2000. PMID: 10899952
-
Effect of a selective 5-HT reuptake inhibitor in combination with 5-HT1A and 5-HT1B receptor antagonists on extracellular 5-HT in rat frontal cortex in vivo.Br J Pharmacol. 1997 Jul;121(5):941-6. doi: 10.1038/sj.bjp.0701235. Br J Pharmacol. 1997. PMID: 9222551 Free PMC article.
-
Potentiation by (-)Pindolol of the activation of postsynaptic 5-HT(1A) receptors induced by venlafaxine.Neuropsychopharmacology. 2000 Sep;23(3):294-306. doi: 10.1016/S0893-133X(00)00112-3. Neuropsychopharmacology. 2000. PMID: 10942853
-
Pharmacology of rapid-onset antidepressant treatment strategies.J Clin Psychiatry. 2001;62 Suppl 15:12-7. J Clin Psychiatry. 2001. PMID: 11444761 Review.
-
Role of 5-HT1A autoreceptors in the mechanism of action of serotoninergic antidepressant drugs: recent findings from in vivo microdialysis studies.Fundam Clin Pharmacol. 1996;10(1):16-27. doi: 10.1111/j.1472-8206.1996.tb00145.x. Fundam Clin Pharmacol. 1996. PMID: 8900496 Review.
Cited by
-
Preclinical characterization of WAY-211612: a dual 5-HT uptake inhibitor and 5-HT (1A) receptor antagonist and potential novel antidepressant.Br J Pharmacol. 2009 May;157(2):307-19. doi: 10.1111/j.1476-5381.2009.00146.x. Epub 2009 Mar 26. Br J Pharmacol. 2009. PMID: 19338583 Free PMC article.
-
Organocatalytic tandem Michael addition reactions: A powerful access to the enantioselective synthesis of functionalized chromenes, thiochromenes and 1,2-dihydroquinolines.Beilstein J Org Chem. 2012;8:1668-94. doi: 10.3762/bjoc.8.191. Epub 2012 Oct 4. Beilstein J Org Chem. 2012. PMID: 23209500 Free PMC article.
-
Neuropharmacology of 5-hydroxytryptamine.Br J Pharmacol. 2006 Jan;147 Suppl 1(Suppl 1):S145-52. doi: 10.1038/sj.bjp.0706427. Br J Pharmacol. 2006. PMID: 16402098 Free PMC article.
-
Strategy to Accelerate or Augment the Antidepressant Response and for An Early Onset of SSRI Activity. Adjunctive Amisulpride to Fluvoxamine in Major Depressive Disorder.Clin Pract Epidemiol Ment Health. 2010 Jan 27;6:1-3. doi: 10.2174/1745017901006010001. Clin Pract Epidemiol Ment Health. 2010. PMID: 20498696 Free PMC article.
-
Effects of amantadine and budipine on antidepressant drug-evoked changes in extracellular 5-HT in the frontal cortex of freely moving rats.Br J Pharmacol. 2005 Jul;145(5):587-92. doi: 10.1038/sj.bjp.0706188. Br J Pharmacol. 2005. PMID: 15834446 Free PMC article.
References
-
- AGHAJANIAN G.K., GRAHAM A.W., SHEARD M.H. Serotonin-containing neurons in brain: depression of firing by monoamine oxidase inhibitors. Science. 1970;169:1100–1102. - PubMed
-
- ANDREWS N., HOGG S., GONZALEZ L.E., FILE S.E. 5-HT1A receptors in the median raphé nucleus and dorsal hippocampus may mediate anxiolytic and anxiogenic behaviours respectively. Eur. J. Pharmacol. 1994;264:259–264. - PubMed
-
- ARTIGAS F., PEREZ V., ALVAREZ E. Pindolol induces a rapid improvement of depressed patients treated with serotonin reuptake inhibitors. Arch. Gen. Psychiatry. 1994;51:248–251. - PubMed
-
- ARTIGAS F., ROMERO L., DE MONTIGNY C., BLIER P. Acceleration of the effect of selected antidepressant drugs in major depression by 5-HT1A antagonists. Trends Neurosci. 1996;19:378–383. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

