Control of glutamate release by calcium channels and kappa-opioid receptors in rodent and primate striatum
- PMID: 10369483
- PMCID: PMC1565998
- DOI: 10.1038/sj.bjp.0702523
Control of glutamate release by calcium channels and kappa-opioid receptors in rodent and primate striatum
Abstract
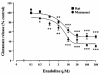
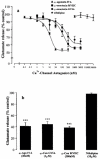
The modulation of depolarization (4-aminopyridine, 2 mM)-evoked endogenous glutamate release by kappa-opioid receptor activation and blockade of voltage-dependent Ca2+ -channels has been investigated in synaptosomes prepared from rat and marmoset striatum. 4-Aminopyridine (4-AP)-stimulated, Ca2+ -dependent glutamate release was inhibited by enadoline, a selective kappa-opioid receptor agonist, in a concentration-dependent and norbinaltorphimine (nor-BNI, selective kappa-opioid receptor antagonist)-sensitive manner in rat (IC50 = 4.4+/-0.4 microM) and marmoset (IC50 = 2.9+/-0.7 microM) striatal synaptosomes. However, in the marmoset, there was a significant (approximately 23%) nor-BNI-insensitive component. In rat striatal synaptosomes, the Ca2+ -channel antagonists omega-agatoxin-IVA (P/Q-type blocker), omega-conotoxin-MVIIC (N/P/Q-type blocker) and omega-conotoxin-GVIA (N-type blocker) reduced 4-AP-stimulated, Ca2+ -dependent glutamate release in a concentration-dependent manner with IC50 values of 6.5+/-0.9 nM, 75.5+5.9 nM and 106.5+/-8.7 nM, respectively. In marmoset striatal synaptosomes, 4-AP-stimulated, Ca2+ -dependent glutamate release was significantly inhibited by omega-agatoxin-IVA (30 nM, 57.6+/-2.3%, inhibition), omega-conotoxin-MVIIC (300 nM, 57.8+/-3.1%) and omega-conotoxin-GVIA (1 microM, 56.7+/-2%). Studies utilizing combinations of Ca2+ -channel antagonists suggests that in the rat striatum, two relatively distinct pools of glutamate, released by activation of either P or Q-type Ca2+ -channels, exist. In contrast, in the primate there is much overlap between the glutamate released by P and Q-type Ca2+ -channel activation. Studies using combinations of enadoline and the Ca2+ -channel antagonists suggest that enadoline-induced inhibition of glutamate release occurs primarily via reduction of Ca2+ -influx through P-type Ca2+ -channels in the rat but via N-type Ca2+ -channels in the marmoset. In conclusion, the results presented suggest that there are species differences in the control of glutamate release by kappa-opioid receptors and Ca2+ -channels.
Figures

Similar articles
-
Effects of N-, P- and Q-type neuronal calcium channel antagonists on mammalian peripheral neurotransmission.Br J Pharmacol. 1996 Sep;119(1):49-56. doi: 10.1111/j.1476-5381.1996.tb15676.x. Br J Pharmacol. 1996. PMID: 8872356 Free PMC article.
-
Presynaptic kappa-opioid and muscarinic receptors inhibit the calcium-dependent component of evoked glutamate release from striatal synaptosomes.J Neurochem. 1999 Sep;73(3):1058-65. doi: 10.1046/j.1471-4159.1999.0731058.x. J Neurochem. 1999. PMID: 10461895
-
Omega-agatoxin-TK is a useful tool to study P-type Ca2+ channel-mediated changes in internal Ca2+ and glutamate release in depolarised brain nerve terminals.Neurochem Int. 2005 Jan;46(1):53-60. doi: 10.1016/j.neuint.2004.07.004. Neurochem Int. 2005. PMID: 15567515
-
Neuroprotective use-dependent blockers of Na+ and Ca2+ channels controlling presynaptic release of glutamate.Ann N Y Acad Sci. 1995 Sep 15;765:210-29. doi: 10.1111/j.1749-6632.1995.tb16578.x. Ann N Y Acad Sci. 1995. PMID: 7486608 Review.
-
Neuroprotective Role of the B Vitamins in the Modulation of the Central Glutamatergic Neurotransmission.CNS Neurol Disord Drug Targets. 2022;21(4):292-301. doi: 10.2174/1871527320666210902165739. CNS Neurol Disord Drug Targets. 2022. PMID: 34477538 Review.
Cited by
-
KOR Control over Addiction Processing: An Exploration of the Mesolimbic Dopamine Pathway.Handb Exp Pharmacol. 2022;271:351-377. doi: 10.1007/164_2020_421. Handb Exp Pharmacol. 2022. PMID: 33301050 Free PMC article.
-
Nalfurafine, the kappa opioid agonist, inhibits icilin-induced wet-dog shakes in rats and antagonizes glutamate release in the dorsal striatum.Neuropharmacology. 2007 Mar;52(3):925-30. doi: 10.1016/j.neuropharm.2006.10.010. Epub 2006 Dec 5. Neuropharmacology. 2007. PMID: 17150231 Free PMC article.
-
Striatal synaptosomes from Hdh140Q/140Q knock-in mice have altered protein levels, novel sites of methionine oxidation, and excess glutamate release after stimulation.J Huntingtons Dis. 2013;2(4):459-75. doi: 10.3233/JHD-130080. J Huntingtons Dis. 2013. PMID: 24696705 Free PMC article.
-
Kappa Opioid Receptors Mediate Heterosynaptic Suppression of Hippocampal Inputs in the Rat Ventral Striatum.J Neurosci. 2017 Jul 26;37(30):7140-7148. doi: 10.1523/JNEUROSCI.0876-17.2017. Epub 2017 Jun 22. J Neurosci. 2017. PMID: 28642282 Free PMC article.
-
Susceptibility to Pentylenetetrazole-Induced Seizures in Mice with Distinct Activity of the Endogenous Opioid System.Int J Mol Sci. 2024 Jun 26;25(13):6978. doi: 10.3390/ijms25136978. Int J Mol Sci. 2024. PMID: 39000086 Free PMC article.
References
-
- ADAMS M.E., MINTZ I.M., REILY M.D., THANABAL V., BEAN B.P. Structure and properties of ω-aga-IVB, a new antagonist of P-type calcium channels. Mol. Pharmac. 1993;44:681–688. - PubMed
-
- ADAMSON P., MANTZOURRIDID T., XIANG J.Z., HAJIMOHAMMADREZA I., BRAMMER M.J., CAMPBELL I.C. α2-Adrenergic, κ-opiate, and P1-purinergic autoreceptors have mutually antagonist effects: a new regulatory mechanism. J. Neurochem. 1989;53:1077–1082. - PubMed
-
- AMBROSIO A.F., MALVA J.O., CARVALHO A.P., CARVALHO C.M. Modulation of Ca2+ channels by activation of adenosine A1 receptors in rat striatal glutamatergic nerve terminals. Neurosci. Lett. 1996;220:163–166. - PubMed
-
- BRADFORD M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilising the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

