Ex vivo assay to determine the cyclooxygenase selectivity of non-steroidal anti-inflammatory drugs
- PMID: 10372826
- PMCID: PMC1565969
- DOI: 10.1038/sj.bjp.0702518
Ex vivo assay to determine the cyclooxygenase selectivity of non-steroidal anti-inflammatory drugs
Abstract
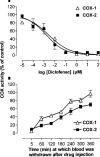
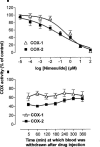
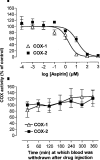
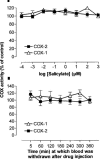
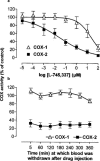
1. In this study we describe experiments to establish ex vivo the selectivity of non-steroidal anti-inflammatory drugs (NSAIDs) given in vivo. 2. Anaesthetised (Inactin, 120 mg kg(-1)) male Wistar rats (220-250 g) received an i.v. dose of one of the following compounds (dose mg kg(-1)): aspirin (20), diclofenac (3), L-745,337 (30), nimesulide (15), salicylate (20), sulindac (10). Blood samples were taken before and up to 6 h after dosing and the plasma obtained from it was tested for its ability to inhibit prostanoid formation in IL-1beta-treated A549 cells (COX-2 system) and human washed platelets (COX-1 system). For control the same compounds were also added directly to the assay systems. 3. All drugs, except sodium salicylate, inhibited COX-1 and COX-2 when added directly to the test systems. Plasma from aspirin-treated rats was without effect on either COX-1 or COX-2, consistent with the rapid in vivo metabolism to salicylate. Conversely, plasma from sulindac-treated rats inhibited COX-1 and COX-2 with potencies according with in vivo metabolism to sulindac sulphide. Diclofenac was COX-1/2 non-selective when tested in vitro, but a slightly preferential inhibitor of COX-2 when tested ex vivo. Nimesulide was confirmed as preferential inhibitor of COX-2 both in vitro and ex vivo. L-745,337 was a selective COX-2 inhibitor when tested in vitro or ex vivo. 4. In conclusion, our experiments show clearly (a) NSAIDs inactivation, (b) activation of prodrugs, and (c) NSAIDs selectivity. Our assay provides useful information about the selectivity of NSAIDs that could be extended by the analysis of plasma samples taken from humans similarly treated with test drugs.
Figures

References
-
- BERNAREGGI A. The pharmacokinetics profile of nimesulide in healthy volunteers. Drugs. 1993;46 suppl. 1:64–72. - PubMed
-
- BRIDEAU C., KARGMAN S., LIU S., DALLOB A.L., EHRICH E.W., RODGER I.W., CHAN C.-C. A human whole blood assay for clinical evaluation of biochemical efficacy of cyclooxygenase inhibitors. Inflamm. Res. 1996;45:68–74. - PubMed
-
- BROUWERS J.R.B.J., DE SMET P.A.G.M. Pharmakokinetic-pharmacodinamic drug interactions with nonsteroidal anti-inflammatory drugs. Clin. Pharmacokinet. 1994;27:462–485. - PubMed
-
- CHAN C.-C., BOYCE S., BRIDEAU C., FORD-HUTCHINSON A.W., GORDON R., GUAY D., HILL R.G., LI C.-S., MANCINI J., PENNETON M., PRASIT P., RASORI R., RIENDEAU D., ROY P., TAGARI P., VICKERS P., WONG E., RODGER W. Pharmacology of a selective cyclooxygenase-2 inhibitor, L-745,337: a novel nonsteroidal anti-inflammatory agent with an ulcerogenic sparing effect in rat and nonhuman primate stomach. J. Pharmacol. Exp. Therap. 1995;274:1531–1537. - PubMed
-
- CROMLISH W.A., KENNEDY B.P. Selective inhibition of cyclooxygenase-1 and -2 using intact insect cell assay. Biochem. Pharmacol. 1996;52:1777–1785. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

