Effects of the endogeneous cannabinoid, anandamide, on neuronal activity in rat hippocampal slices
- PMID: 10372827
- PMCID: PMC1565956
- DOI: 10.1038/sj.bjp.0702478
Effects of the endogeneous cannabinoid, anandamide, on neuronal activity in rat hippocampal slices
Abstract
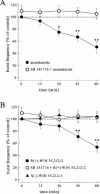
1. The arachidonic acid derivative arachidonylethanolamide (anandamide) is an endogeneous ligand of cannabinoid receptors that induces pharmacological actions similar to those of cannabinoids such as delta9-tetrahydrocannabinol (THC). We examined whether anandamide can influence excessive neuronal activity by investigating stimulation-induced population spikes and epileptiform activity in rat hippocampal slices. For this purpose, the effects of anandamide were compared with those of the synthetic cannabinoid agonist WIN 55,212-2 and its inactive S(-)-enantiomer WIN 55,212-3. 2. Both anandamide (1 and 10 microM) and WIN 55,212-2 (0.1 and 1 microM) decreased the amplitude of the postsynaptic population spike and the slope of the field excitatory postsynaptic potential (field e.p.s.p.) without affecting the presynaptic fibre spike of the afferents. At a concentration of 1 microM, WIN 55,212-2 completely suppressed the postsynaptic spike, whereas the S(-)-enantiomer WIN 55,212-3 produced only a slight depression. The CB1 receptor antagonist SR 141716 blocked the inhibition evoked by the cannabinoids. SR 141716 had a slight facilitatory effect on neuronal excitability by itself. 3. Anandamide shifted the input-output curve of the postsynaptic spike and the field e.p.s.p. to the right and increased the magnitude of paired-pulse facilitation indicating a presynaptic mechanism of action. 4. Anandamide and WIN 55,212-2, but not WIN 55,212-3, attenuated both stimulus-triggered epileptiform activity in CA1 elicited by omission of Mg2+ and spontaneously occurring epileptiform activity in CA3 elicited by omission of Mg2+ and elevation of K+ to 8 mM. The antiepileptiform effect of these cannabinoids was blocked by SR 141716. 5. In conclusion, cannabinoid receptors of the CB1 type as well as their endogeneous ligand, anandamide, are involved in the control of neuronal excitability, thus reducing excitatory neurotransmission at a presynaptic site, a mechanism which might be involved in the prevention of excessive excitability leading to epileptiform activity.
Figures






References
-
- ABOOD M.E., MARTIN B.R. Molecular neurobiology of the cannabinoid receptor. Int. Rev. Neurobiol. 1996;39:197–221. - PubMed
-
- ANDERSON W.W., LEWIS D.V., SWARTZWELDER H.S., WILSON W.A. Magnesium-free medium activates seizure events in rat hippocampal slices. Brain Res. 1986;398:215–219. - PubMed
-
- APLAND J.P., CANN F. Anticonvulsant effects of memantine and MK-801 in guinea pig hippocampal slices. Brain Res. Bull. 1995;37:311–316. - PubMed
-
- BIDAUT-RUSSELL M., DEVANNE W.A., HOWLETT A.C. Cannabinoid receptors and modulation of cyclic AMP in the rat brain. J. Neurochem. 1990;55:21–26. - PubMed
-
- BOUABOULA M., PERRACHON S., MILLIGAN L., CANAT X., RINALDI-CARMONA M., PORTIER M., BARTH F., CALANDRA B., PECCEU F., LUPKER J., MAFFRAND J.-P., LE FUR G., CASELLAS P. A selective inverse agonist for central cannabinoid receptor inhibits mitogen-activated protein kinase activation stimulated by insulin or insulin-like growth factor 1. Evidence for a new model of receptor/ligand interactions. J. Biol. Chem. 1997;272:22330–22339. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

