Ras-specific exchange factor GRF: oligomerization through its Dbl homology domain and calcium-dependent activation of Raf
- PMID: 10373510
- PMCID: PMC84259
- DOI: 10.1128/MCB.19.7.4611
Ras-specific exchange factor GRF: oligomerization through its Dbl homology domain and calcium-dependent activation of Raf
Abstract
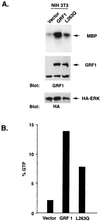
The full-length versions of the Ras-specific exchange factors Ras-GRF1 (GRF1) and Ras-GRF2 (GRF2), which are expressed in brain and a restricted number of other organs, possess an ionomycin-dependent activation of Erk mitogen-activated protein kinase activity in 293T cells (C. L. Farnsworth et al., Nature 376:524-527, 1995; N. P. Fam et al., Mol. Cell. Biol. 17:1396-1406, 1996). Each GRF protein contains a Dbl homology (DH) domain. A yeast two-hybrid screen was used to identify polypeptides that associate with the DH domain of GRF1. In this screen, a positive cDNA clone from a human brain cDNA library was isolated which consisted of the GRF2 DH domain and its adjacent ilimaquinone domain. Deletion analysis verified that the two-hybrid interaction required only the DH domains, and mutation of Leu-263 to Gln (L263Q) in the N terminus of the GRF1 DH domain abolished the two-hybrid interaction, while a cluster of more C-terminally located mutations in the DH domain did not eliminate the interaction. Oligomers between GRF1 and GRF2 were detected in a rat brain extract, and forced expression of GRF1 and GRF2 in cultured mammalian cells formed homo- and hetero-oligomers. Introduction of the L263Q mutation in GRF1 led to a protein that was deficient in oligomer formation, while GRF1 containing the DH cluster mutations formed homo-oligomers with an efficiency similar to that of wild type. Compared to wild-type GRF1, the focus-forming activity on NIH 3T3 cells of the GRF1 DH cluster mutant was reduced, while the L263Q mutant was inactive. Both mutants were impaired in their ability to mediate ionomycin-dependent Erk activity in 293T cells. In the absence of ionomycin, 293T cells expressing wild-type GRF1 contained much higher levels of Ras-GTP than control cells; the increase in Erk activity induced by ionomycin in the GRF1-expressing cells also induced a concomitant increase in Raf kinase activity, but without a further increase in the level Ras-GTP. We conclude that GRF1 and GRF2 can form homo- and hetero-oligomers via their DH domains, that mutational inactivation of oligomer formation by GRF1 is associated with impaired biological and signaling activities, and that in 293T cells GRF1 mediates at least two pathways for Raf activation: one a constitutive signal that is mainly Ras-dependent, and one an ionomycin-induced signal that cooperates with the constitutive signal without further augmenting the level of GTP-Ras.
Figures




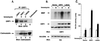
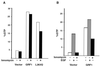

References
-
- Baouz S, Jacquet E, Bernardi A, Parmeggiani A. The N-terminal moiety of CDC25Mm, a GDP/GTP exchange factor of Ras proteins, controls the activity of the catalytic domain: modulation by calmodulin and calpain. J Biol Chem. 1997;272:6671–6676. - PubMed
-
- Boguski M S, McCormick F. Proteins regulating Ras and its relatives. Nature. 1993;366:643–654. - PubMed
-
- Brambilla R, Gnesutta N, Minichiello L, White G, Roylance A J, Herron C E, Ramsey M, Wolfer D P, Cestari V, Rossi-Arnaud C, Grant S G N, Chapman P F, Lipp H P, Sturani E, Klein R. A role for the Ras signalling pathway in synaptic transmission and long-term memory. Nature. 1997;390:281–286. - PubMed
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
