c-Myc regulates cyclin D-Cdk4 and -Cdk6 activity but affects cell cycle progression at multiple independent points
- PMID: 10373516
- PMCID: PMC84265
- DOI: 10.1128/MCB.19.7.4672
c-Myc regulates cyclin D-Cdk4 and -Cdk6 activity but affects cell cycle progression at multiple independent points
Abstract
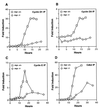
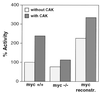
c-myc is a cellular proto-oncogene associated with a variety of human cancers and is strongly implicated in the control of cellular proliferation, programmed cell death, and differentiation. We have previously reported the first isolation of a c-myc-null cell line. Loss of c-Myc causes a profound growth defect manifested by the lengthening of both the G1 and G2 phases of the cell cycle. To gain a clearer understanding of the role of c-Myc in cellular proliferation, we have performed a comprehensive analysis of the components that regulate cell cycle progression. The largest defect observed in c-myc-/- cells is a 12-fold reduction in the activity of cyclin D1-Cdk4 and -Cdk6 complexes during the G0-to-S transition. Downstream events, such as activation of cyclin E-Cdk2 and cyclin A-Cdk2 complexes, are delayed and reduced in magnitude. However, it is clear that c-Myc affects the cell cycle at multiple independent points, because restoration of the Cdk4 and -6 defect does not significantly increase growth rate. In exponentially cycling cells the absence of c-Myc reduces coordinately the activities of all cyclin-cyclin-dependent kinase complexes. An analysis of cyclin-dependent kinase complex regulators revealed increased expression of p27(KIP1) and decreased expression of Cdk7 in c-myc-/- cells. We propose that c-Myc functions as a crucial link in the coordinate adjustment of growth rate to environmental conditions.
Figures




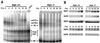


Similar articles
-
Transforming growth factor beta(1) selectively inhibits the cyclic AMP-dependent proliferation of primary thyroid epithelial cells by preventing the association of cyclin D3-cdk4 with nuclear p27(kip1).Mol Biol Cell. 2000 Mar;11(3):1061-76. doi: 10.1091/mbc.11.3.1061. Mol Biol Cell. 2000. PMID: 10712520 Free PMC article.
-
Expression and activity of the retinoblastoma protein (pRB)-family proteins, p107 and p130, during L6 myoblast differentiation.Cell Growth Differ. 1995 Oct;6(10):1287-98. Cell Growth Differ. 1995. PMID: 8845306
-
Cell cycle exit during terminal erythroid differentiation is associated with accumulation of p27(Kip1) and inactivation of cdk2 kinase.Blood. 2000 Oct 15;96(8):2746-54. Blood. 2000. PMID: 11023508
-
[Molecular mechanisms controlling the cell cycle: fundamental aspects and implications for oncology].Cancer Radiother. 2001 Apr;5(2):109-29. doi: 10.1016/s1278-3218(01)00087-7. Cancer Radiother. 2001. PMID: 11355576 Review. French.
-
Cell cycle and transcriptional control of human myeloid leukemic cells by transforming growth factor beta.Leuk Lymphoma. 2000 Jul;38(3-4):235-46. doi: 10.3109/10428190009087015. Leuk Lymphoma. 2000. PMID: 10830731 Review.
Cited by
-
Wnt9a Is Required for the Aortic Amplification of Nascent Hematopoietic Stem Cells.Cell Rep. 2016 Nov 1;17(6):1595-1606. doi: 10.1016/j.celrep.2016.10.027. Cell Rep. 2016. PMID: 27806298 Free PMC article.
-
Identification of a novel E-box binding pyrrole-imidazole polyamide inhibiting MYC-driven cell proliferation.Cancer Sci. 2015 Apr;106(4):421-9. doi: 10.1111/cas.12610. Epub 2015 Mar 3. Cancer Sci. 2015. PMID: 25611295 Free PMC article.
-
Lnc HAGLR Promotes Colon Cancer Progression Through Sponging miR-185-5p and Activating CDK4 and CDK6 in vitro and in vivo.Onco Targets Ther. 2020 Jun 22;13:5913-5925. doi: 10.2147/OTT.S246092. eCollection 2020. Onco Targets Ther. 2020. PMID: 32606801 Free PMC article.
-
Control of cellular senescence by CPEB.Genes Dev. 2006 Oct 1;20(19):2701-12. doi: 10.1101/gad.1438906. Genes Dev. 2006. PMID: 17015432 Free PMC article.
-
Cytokine signaling to the cell cycle.Immunol Res. 2007;39(1-3):173-84. doi: 10.1007/s12026-007-0080-5. Immunol Res. 2007. PMID: 17917064 Review.
References
-
- Amati B, Brooks M W, Levy N, Littlewood T D, Evan G I, Land H. Oncogenic activity of the c-Myc protein requires dimerization with Max. Cell. 1993;72:233–245. - PubMed
-
- Amati B, Dalton S, Brooks M W, Littlewood T D, Evan G I, Land H. Transcriptional activation by the human c-Myc oncoprotein in yeast requires interaction with Max. Nature. 1992;359:423–426. - PubMed
-
- Bates S, Bonetta L, Macallan D, Parry D, Holder A, Dickson C, Peters G. CDK6 (PLSTIRE) and CDK4 (PSKJ3) are a distinct subset of the cyclin-dependent kinases that associate with cyclin D1. Oncogene. 1994;9:71–79. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
