p70(s6k) integrates phosphatidylinositol 3-kinase and rapamycin-regulated signals for E2F regulation in T lymphocytes
- PMID: 10373522
- PMCID: PMC84271
- DOI: 10.1128/MCB.19.7.4729
p70(s6k) integrates phosphatidylinositol 3-kinase and rapamycin-regulated signals for E2F regulation in T lymphocytes
Abstract
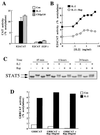
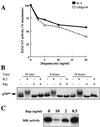
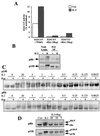
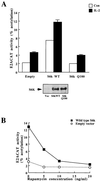
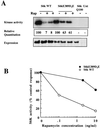
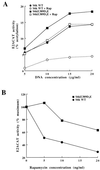
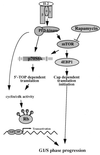
In T lymphocytes, the hematopoietic cytokine interleukin-2 (IL-2) uses phosphatidylinositol 3-kinase (PI 3-kinase)-induced signaling pathways to regulate E2F transcriptional activity, a critical cell cycle checkpoint. PI 3-kinase also regulates the activity of p70(s6k), the 40S ribosomal protein S6 kinase, a response that is abrogated by the macrolide rapamycin. This immunosuppressive drug is known to prevent T-cell proliferation, but the precise point at which rapamycin regulates T-cell cycle progression has yet to be elucidated. Moreover, the effects of rapamycin on, and the role of p70(s6k) in, IL-2 and PI 3-kinase activation of E2Fs have not been characterized. Our present results show that IL-2- and PI 3-kinase-induced pathways for the regulation of E2F transcriptional activity include both rapamycin-resistant and rapamycin-sensitive components. Expression of a rapamycin-resistant mutant of p70(s6k) in T cells could restore rapamycin-suppressed E2F responses. Thus, the rapamycin-controlled processes involved in E2F regulation appear to be mediated by p70(s6k). However, the rapamycin-resistant p70(s6k) could not rescue rapamycin inhibition of T-cell cycle entry, consistent with the involvement of additional, rapamycin-sensitive pathways in the control of T-cell cycle progression. The present results thus show that p70(s6k) is able to regulate E2F transcriptional activity and provide direct evidence for the first time for a link between IL-2 receptors, PI 3-kinase, and p70(s6k) that regulates a crucial G1 checkpoint in T lymphocytes.
Figures


References
-
- Brennan P, Babbage J W, Burgering B M T, Groner B, Reif K, Cantrell D A. Phosphatidylinositol 3-kinase controls E2F transcriptional activity in response to interleukin-2. Immunity. 1997;7:679–689. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
