Inhibition of double-stranded RNA- and tumor necrosis factor alpha-mediated apoptosis by tetratricopeptide repeat protein and cochaperone P58(IPK)
- PMID: 10373525
- PMCID: PMC84274
- DOI: 10.1128/MCB.19.7.4757
Inhibition of double-stranded RNA- and tumor necrosis factor alpha-mediated apoptosis by tetratricopeptide repeat protein and cochaperone P58(IPK)
Abstract
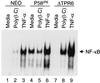
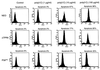
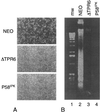
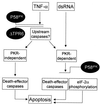
P58(IPK) is a tetratricopeptide repeat-containing cochaperone that is involved in stress-activated cellular pathways and that inhibits the activity of protein kinase PKR, a primary mediator of the antiviral and antiproliferative properties of interferon. To gain better insight into the molecular actions of P58(IPK), we generated NIH 3T3 cell lines expressing either wild-type P58(IPK) or a P58(IPK) deletion mutant, DeltaTPR6, that does not bind to or inhibit PKR. When treated with double-stranded RNA (dsRNA), DeltaTPR6-expressing cells exhibited a significant increase in eukaryotic initiation factor 2alpha phosphorylation and NF-kappaB activation, indicating a functional PKR. In contrast, both of these PKR-dependent events were blocked by the overexpression of wild-type P58(IPK). In addition, the P58(IPK) cell line, but not the DeltaTPR6 cell line, was resistant to dsRNA-induced apoptosis. Together, these findings demonstrate that P58(IPK) regulates dsRNA signaling pathways by inhibiting multiple PKR-dependent functions. In contrast, both the P58(IPK) and DeltaTPR6 cell lines were resistant to tumor necrosis factor alpha-induced apoptosis, suggesting that P58(IPK) may function as a more general suppressor of programmed cell death independently of its PKR-inhibitory properties. In accordance with this hypothesis, although PKR remained active in DeltaTPR6-expressing cells, the DeltaTPR6 cell line displayed a transformed phenotype and was tumorigenic in nude mice. Thus, the antiapoptotic function of P58(IPK) may be an important factor in its ability to malignantly transform cells.
Figures
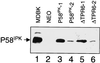



References
-
- Abraham N, Stojdl D F, Duncan P I, Méthot N, Ishii T, Dubé M, Vanderhyden B C, Atkins H L, Gray D A, McBurney M W, Koromilas A E, Brown E G, Sonenberg N, Bell J C. Characterization of transgenic mice with targeted disruption of the catalytic domain of the double-stranded RNA-dependent protein kinase, PKR. J Biol Chem. 1999;274:5953–5962. - PubMed
-
- Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1988.
-
- Barber G N, Jagus R, Meurs E F, Hovanessian A G, Katze M G. Molecular mechanisms responsible for malignant transformation by regulatory and catalytic domain variants of the interferon-induced enzyme RNA-dependent protein kinase. J Biol Chem. 1995;270:17423–17428. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
