Tyrosine phosphorylation of the proto-oncoprotein Raf-1 is regulated by Raf-1 itself and the phosphatase Cdc25A
- PMID: 10373531
- PMCID: PMC84280
- DOI: 10.1128/MCB.19.7.4819
Tyrosine phosphorylation of the proto-oncoprotein Raf-1 is regulated by Raf-1 itself and the phosphatase Cdc25A
Abstract
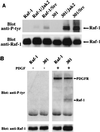
There is a growing body of evidence demonstrating that Raf-1 is phosphorylated on tyrosines upon stimulation of a variety of receptors. Although detection of Raf-1 tyrosine phosphorylation has remained elusive, genetic analyses have demonstrated it to be important for Raf-1 activation. Here we report new findings which indicate that Raf-1 tyrosine phosphorylation is regulated in vivo. In both a mammalian and baculovirus expression system, a kinase-inactive allele of Raf-1 was found to be tyrosine phosphorylated at levels much greater than that of wild-type Raf-1. The level of tyrosine phosphate on Raf-1 was markedly increased upon treatment with phosphatase inhibitors either before or after cell lysis. Cdc25A was found to dephosphorylate Raf-1 on tyrosines that resulted in a significant decrease in Raf-1 kinase activity. In NIH 3T3 cells, coexpression of wild-type Raf-1 and phosphatase-inactive Cdc25A led to a marked increase in Raf-1 tyrosine phosphorylation in response to platelet-derived growth factor. These data suggest that the tyrosine phosphorylation of Raf-1 is regulated not only by itself but also by Cdc25A.
Figures

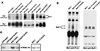
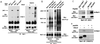
Similar articles
-
A dominant role for the Raf-MEK pathway in forskolin, 12-O-tetradecanoyl-phorbol acetate, and platelet-derived growth factor-induced CREB (cAMP-responsive element-binding protein) activation, uncoupled from serine 133 phosphorylation in NIH 3T3 cells.Mol Endocrinol. 1999 Jul;13(7):1071-83. doi: 10.1210/mend.13.7.0293. Mol Endocrinol. 1999. PMID: 10406459
-
Activation of the Raf-1/MEK/Erk kinase pathway by a novel Cdc25 inhibitor in human prostate cancer cells.Prostate. 2004 Jan 1;58(1):95-102. doi: 10.1002/pros.10292. Prostate. 2004. PMID: 14673957
-
Interleukin 2 regulates Raf-1 kinase activity through a tyrosine phosphorylation-dependent mechanism in a T-cell line.Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5544-8. doi: 10.1073/pnas.90.12.5544. Proc Natl Acad Sci U S A. 1993. PMID: 7685905 Free PMC article.
-
Feedback regulation of Raf-1 and mitogen-activated protein kinase (MAP) kinase kinases 1 and 2 by MAP kinase phosphatase-1 (MKP-1).J Biol Chem. 1998 Jan 16;273(3):1788-93. doi: 10.1074/jbc.273.3.1788. J Biol Chem. 1998. PMID: 9430728
-
The Croonian Lecture 1997. The phosphorylation of proteins on tyrosine: its role in cell growth and disease.Philos Trans R Soc Lond B Biol Sci. 1998 Apr 29;353(1368):583-605. doi: 10.1098/rstb.1998.0228. Philos Trans R Soc Lond B Biol Sci. 1998. PMID: 9602534 Free PMC article. Review.
Cited by
-
Targeting 14-3-3ε-CDC25A interactions to trigger apoptotic cell death in skin cancer.Oncotarget. 2020 Sep 1;11(35):3267-3278. doi: 10.18632/oncotarget.27700. eCollection 2020 Sep 1. Oncotarget. 2020. PMID: 32934772 Free PMC article.
-
CDC25A phosphatase controls meiosis I progression in mouse oocytes.Dev Biol. 2008 May 1;317(1):260-9. doi: 10.1016/j.ydbio.2008.02.028. Epub 2008 Mar 4. Dev Biol. 2008. PMID: 18367163 Free PMC article.
-
The cell cycle-regulatory CDC25A phosphatase inhibits apoptosis signal-regulating kinase 1.Mol Cell Biol. 2001 Jul;21(14):4818-28. doi: 10.1128/MCB.21.14.4818-4828.2001. Mol Cell Biol. 2001. PMID: 11416155 Free PMC article.
-
Kizuna is a novel mitotic substrate for CDC25B phosphatase.Cell Cycle. 2014;13(24):3867-77. doi: 10.4161/15384101.2014.972882. Cell Cycle. 2014. PMID: 25558830 Free PMC article.
-
Multimodal control of Cdc25A by nitrosative stress.Cancer Res. 2008 Sep 15;68(18):7457-65. doi: 10.1158/0008-5472.CAN-08-0625. Cancer Res. 2008. PMID: 18794133 Free PMC article.
References
-
- Barber D L, Corless C N, Xia K, Roberts T M, D’Andrea A D. Erythropoietin activates Raf1 by an Shc-independent pathway in CTLL-EPO-R cells. Blood. 1997;89:55–64. - PubMed
-
- Carroll M P, Clark-Lewis I, Rapp U R, May W S. Interleukin-3 and granulocyte-macrophage colony-stimulating factor mediate rapid phosphorylation and activation of cytosolic c-raf. J Biol Chem. 1990;265:19812–19817. - PubMed
-
- Dent P, Jelinek T, Morrison D K, Weber M J, Sturgill T W. Reversal of Raf-1 activation by purified and membrane-associated protein phosphatases. Science. 1995;268:1902–1906. . (Erratum, 269:1657.) - PubMed
-
- Dent P, Reardon D B, Wood S L, Lindorfer M A, Graber S G, Garrison J C, Brautigan D L, Sturgill T W. Inactivation of raf-1 by a protein-tyrosine phosphatase stimulated by GTP and reconstituted by Galphai/o subunits. J Biol Chem. 1996;271:3119–3123. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
