Repressive effect of Sp1 on the C/EBPalpha gene promoter: role in adipocyte differentiation
- PMID: 10373535
- PMCID: PMC84284
- DOI: 10.1128/MCB.19.7.4855
Repressive effect of Sp1 on the C/EBPalpha gene promoter: role in adipocyte differentiation
Abstract
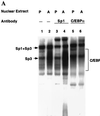
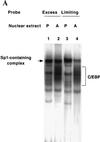
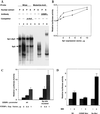
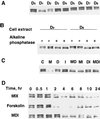
Expression of C/EBPalpha is required for differentiation of 3T3-L1 preadipocytes into adipocytes. Previous investigations indicated that transcription of the C/EBPalpha gene is sequentially activated during differentiation, initially by C/EBPbeta and C/EBPdelta and later by C/EBPalpha (autoactivation). These events are mediated by a C/EBP regulatory element in the promoter of the C/EBPalpha gene. This article presents evidence that members of the Sp family, notably Sp1, act repressively on the C/EBPalpha promoter prior to the induction of differentiation. Sp1 was shown to bind to a GC box at the 5' end of the C/EBP regulatory element in the C/EBPalpha promoter and, in so doing, to competitively prevent binding to and transactivation of the promoter by the C/EBPs. One of the differentiation inducers methylisobutylxanthine (a cAMP phosphodiesterase inhibitor) or Forskolin, both of which increase the cellular cAMP level, causes down-regulation of Sp1. This decrease in Sp1 level early in the differentiation program appears to facilitate access of C/EBPbeta and/or C/EBPdelta to the C/EBP regulatory element and, thereby, derepression of the C/EBPalpha gene.
Figures









References
-
- Bernlohr D A, Bolanowski M A, Kelly T J, Lane M D. Evidence for an increase in transcription of specific mRNAs during differentiation of 3T3-L1 preadipocytes. J Biol Chem. 1985;260:5563–5567. - PubMed
-
- Cao Z, Umek R M, McKnight S L. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. 1991;5:1538–1552. - PubMed
-
- Christy R J, Yang V W, Ntambi J M, Geiman D E, Landschulz W H, Friedman A D, Nakabeppu Y, Kelly T J, Lane M D. Differentiation-induced gene expression in 3T3-L1 preadipocytes: CCAAT/enhancer binding protein interacts with and activates the promoters of two adipocyte-specific genes. Genes Dev. 1989;3:1323–1335. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
