Disordered T-cell development and T-cell malignancies in SCL LMO1 double-transgenic mice: parallels with E2A-deficient mice
- PMID: 10373552
- PMCID: PMC84335
- DOI: 10.1128/MCB.19.7.5025
Disordered T-cell development and T-cell malignancies in SCL LMO1 double-transgenic mice: parallels with E2A-deficient mice
Abstract
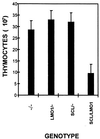
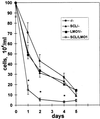
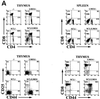
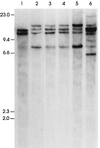
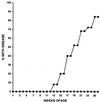
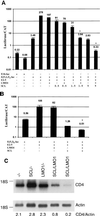
The gene most commonly activated by chromosomal rearrangements in patients with T-cell acute lymphoblastic leukemia (T-ALL) is SCL/tal. In collaboration with LMO1 or LMO2, the thymic expression of SCL/tal leads to T-ALL at a young age with a high degree of penetrance in transgenic mice. We now show that SCL LMO1 double-transgenic mice display thymocyte developmental abnormalities in terms of proliferation, apoptosis, clonality, and immunophenotype prior to the onset of a frank malignancy. At 4 weeks of age, thymocytes from SCL LMO1 mice show 70% fewer total thymocytes, with increased rates of both proliferation and apoptosis, than control thymocytes. At this age, a clonal population of thymocytes begins to populate the thymus, as evidenced by oligoclonal T-cell-receptor gene rearrangements. Also, there is a dramatic increase in immature CD44(+) CD25(-) cells, a decrease in the more mature CD4(+) CD8(+) cells, and development of an abnormal CD44(+) CD8(+) population. An identical pattern of premalignant changes is seen with either a full-length SCL protein or an amino-terminal truncated protein which lacks the SCL transactivation domain, demonstrating that the amino-terminal portion of SCL is not important for leukemogenesis. Lastly, we show that the T-ALL which develop in the SCL LMO1 mice are strikingly similar to those which develop in E2A null mice, supporting the hypothesis that SCL exerts its oncogenic action through a functional inactivation of E proteins.
Figures





References
-
- Aplan P D, Lombardi D P, Ginsberg A M, Cossman J, Bertness V I, Kirsch I R. Disruption of the human SCL locus by “illegitimate” V-(D)-J recombinase activity. Science. 1990;250:1426–1429. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
