Distinct glucocorticoid receptor transcriptional regulatory surfaces mediate the cytotoxic and cytostatic effects of glucocorticoids
- PMID: 10373553
- PMCID: PMC84339
- DOI: 10.1128/MCB.19.7.5036
Distinct glucocorticoid receptor transcriptional regulatory surfaces mediate the cytotoxic and cytostatic effects of glucocorticoids
Abstract
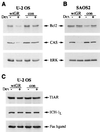
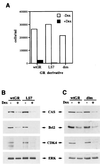
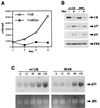
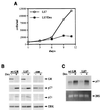
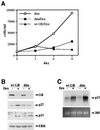
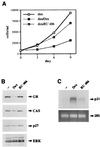
Glucocorticoids act through the glucocorticoid receptor (GR), which can function as a transcriptional activator or repressor, to elicit cytostatic and cytotoxic effects in a variety of cells. The molecular mechanisms regulating these events and the target genes affected by the activated receptor remain largely undefined. Using cultured human osteosarcoma cells as a model for the GR antiproliferative effect, we demonstrate that in U20S cells, GR activation leads to irreversible growth inhibition, apoptosis, and repression of Bcl2. This cytotoxic effect is mediated by GR's transcriptional repression function, since transactivation-deficient mutants and ligands still bring about apoptosis and Bcl2 down-regulation. In contrast, the antiproliferative effect of GR in SAOS2 cells is reversible, does not result in apoptosis or repression of Bcl2, and is a function of the receptor's ability to stimulate transcription. Thus, the cytotoxic versus cytostatic outcome of glucocorticoid treatment is cell context dependent. Interestingly, the cytostatic effect of glucocorticoids in SAOS2 cells involves multiple GR activation surfaces. GR mutants and ligands that disrupt individual transcriptional activation functions (activation function 1 [AF-1] and AF-2) or receptor dimerization fail to fully inhibit cellular proliferation and, remarkably, discriminate between the targets of GR's cytostatic action, the cyclin-dependent kinase inhibitors p21(Cip1) and p27(Kip1). Induction of p21(Cip1) is agonist dependent and requires AF-2 but not AF-1 or GR dimerization. In contrast, induction of p27(Kip1) is agonist independent, does not require AF-2 or AF-1, but depends on GR dimerization. Our findings indicate that multiple GR transcriptional regulatory mechanisms that employ distinct receptor surfaces are used to evoke either the cytostatic or cytotoxic response to glucocorticoids.
Figures




Similar articles
-
Glucocorticoid receptor-mediated cell cycle arrest is achieved through distinct cell-specific transcriptional regulatory mechanisms.Mol Cell Biol. 1997 Jun;17(6):3181-93. doi: 10.1128/MCB.17.6.3181. Mol Cell Biol. 1997. PMID: 9154817 Free PMC article.
-
Glucocorticoids stimulate p21(CIP1) in mesangial cells and in anti-GBM glomerulonephritis.Kidney Int. 2001 May;59(5):1706-16. doi: 10.1046/j.1523-1755.2001.0590051706.x. Kidney Int. 2001. PMID: 11318941
-
1,25-Dihydroxyvitamin D3 induces differentiation of a retinoic acid-resistant acute promyelocytic leukemia cell line (UF-1) associated with expression of p21(WAF1/CIP1) and p27(KIP1).Blood. 1999 Apr 1;93(7):2225-33. Blood. 1999. PMID: 10090931
-
How to tame your genes: mechanisms of inflammatory gene repression by glucocorticoids.FEBS Lett. 2022 Oct;596(20):2596-2616. doi: 10.1002/1873-3468.14409. Epub 2022 Jun 13. FEBS Lett. 2022. PMID: 35612756 Review.
-
The Biologist's Guide to the Glucocorticoid Receptor's Structure.Cells. 2023 Jun 15;12(12):1636. doi: 10.3390/cells12121636. Cells. 2023. PMID: 37371105 Free PMC article. Review.
Cited by
-
Target-specific utilization of transcriptional regulatory surfaces by the glucocorticoid receptor.Proc Natl Acad Sci U S A. 2003 Nov 25;100(24):13845-50. doi: 10.1073/pnas.2336092100. Epub 2003 Nov 14. Proc Natl Acad Sci U S A. 2003. PMID: 14617768 Free PMC article.
-
Modulation of transcription parameters in glucocorticoid receptor-mediated repression.Mol Cell Endocrinol. 2008 Nov 25;295(1-2):59-69. doi: 10.1016/j.mce.2008.05.008. Epub 2008 May 21. Mol Cell Endocrinol. 2008. PMID: 18583028 Free PMC article.
-
Pro-apoptotic protein BIM in apoptosis of glucocorticoid-sensitive and -resistant acute lymphoblastic leukemia CEM cells.Med Oncol. 2011 Dec;28(4):1609-17. doi: 10.1007/s12032-010-9641-x. Epub 2010 Aug 10. Med Oncol. 2011. PMID: 20697841
-
Antiprogestin mifepristone inhibits the growth of cancer cells of reproductive and non-reproductive origin regardless of progesterone receptor expression.BMC Cancer. 2011 May 27;11:207. doi: 10.1186/1471-2407-11-207. BMC Cancer. 2011. PMID: 21619605 Free PMC article.
-
Thiazolidinediones are partial agonists for the glucocorticoid receptor.Endocrinology. 2009 Jan;150(1):75-86. doi: 10.1210/en.2008-0196. Epub 2008 Sep 18. Endocrinology. 2009. PMID: 18801908 Free PMC article.
References
-
- Adams J M, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1326. - PubMed
-
- Alam J, Cook J L. Reporter genes: application to the study of mammalian gene transcription. Anal Biochem. 1990;188:245–254. - PubMed
-
- Alessandrini A, Chiaur D S, Pagano M. Regulation of the cyclin-dependent kinase inhibitor p27 by degradation and phosphorylation. Leukemia. 1997;11:342–345. - PubMed
-
- Alnemri E S, Fernandes T F, Haldar S, Croce C M, Litwack G. Involvement of Bcl-2 in glucocorticoid-induced apoptosis of human pre-B-leukemias. Cancer Res. 1992;52:491–495. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
