Domain swapping used to investigate the mechanism of protein kinase B regulation by 3-phosphoinositide-dependent protein kinase 1 and Ser473 kinase
- PMID: 10373555
- PMCID: PMC84347
- DOI: 10.1128/MCB.19.7.5061
Domain swapping used to investigate the mechanism of protein kinase B regulation by 3-phosphoinositide-dependent protein kinase 1 and Ser473 kinase
Abstract
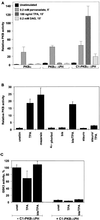
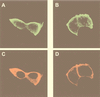
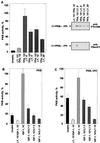
Protein kinase B (PKB or Akt), a downstream effector of phosphoinositide 3-kinase (PI 3-kinase), has been implicated in insulin signaling and cell survival. PKB is regulated by phosphorylation on Thr308 by 3-phosphoinositide-dependent protein kinase 1 (PDK1) and on Ser473 by an unidentified kinase. We have used chimeric molecules of PKB to define different steps in the activation mechanism. A chimera which allows inducible membrane translocation by lipid second messengers that activate in vivo protein kinase C and not PKB was created. Following membrane attachment, the PKB fusion protein was rapidly activated and phosphorylated at the two key regulatory sites, Ser473 and Thr308, in the absence of further cell stimulation. This finding indicated that both PDK1 and the Ser473 kinase may be localized at the membrane of unstimulated cells, which was confirmed for PDK1 by immunofluorescence studies. Significantly, PI 3-kinase inhibitors prevent the phosphorylation of both regulatory sites of the membrane-targeted PKB chimera. Furthermore, we show that PKB activated at the membrane was rapidly dephosphorylated following inhibition of PI 3-kinase, with Ser473 being a better substrate for protein phosphatase. Overall, the results demonstrate that PKB is stringently regulated by signaling pathways that control both phosphorylation/activation and dephosphorylation/inactivation of this pivotal protein kinase.
Figures






References
-
- Alessi D R, James S R, Downes C P, Holmes A B, Gaffney P R J, Reese C, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Bα. Curr Biol. 1997;7:261–269. - PubMed
-
- Alessi D R, Deak M, Casamayor A, Caudwell F B, Morrice N, Norman D G, Gaffney P, Reese C B, MacDoucall C N, Harbison D, Ashwarth A, Bownes M. 3-Phosphoinositide-dependent protein kinase-1 (PDK1): structural and functional homology with the Drosophila DSTPK61 kinase. Curr Biol. 1997;7:776–789. - PubMed
-
- Andersson S, Davie D N, Dahlbäck H, Jörnvall H, Russell D W. Cloning, structure, and expression of the mitochondrial cytochrome P-450 sterol 26-hydroxylase, a bile-acid biosynthetic enzyme. J Biol Chem. 1989;264:8222–8229. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
