Cell cycle withdrawal promotes myogenic induction of Akt, a positive modulator of myocyte survival
- PMID: 10373556
- PMCID: PMC84350
- DOI: 10.1128/MCB.19.7.5073
Cell cycle withdrawal promotes myogenic induction of Akt, a positive modulator of myocyte survival
Abstract
During myogenesis, proliferating myoblasts withdraw from the cell cycle, acquire an apoptosis-resistant phenotype, and differentiate into myotubes. Previous studies indicate that myogenic induction of the cyclin-dependent kinase inhibitor p21 results in an inhibition of apoptotic cell death in addition to its role as a negative cell cycle regulator. Here we demonstrate that the protein encoded by the Akt proto-oncogene is induced in C2C12 cells during myogenic differentiation with a corresponding increase in kinase activity. In differentiating cultures, expression of dominant-negative forms of Akt increase the frequency of cell death whereas expression of wild-type Akt protects against death, indicating that Akt is a positive modulator of myocyte survival. Antisense oligonucleotides against p21 block cell cycle withdrawal, inhibit Akt induction, and enhance cell death in differentiating myocyte cultures. Adenovirus-mediated transfer of wild-type or constitutively active Akt constructs confer partial resistance to cell death under conditions where cell cycle exit is blocked by the antisense oligonucleotides. Collectively, these data indicate that cell cycle withdrawal facilitates the induction of Akt during myogenesis, promoting myocyte survival.
Figures




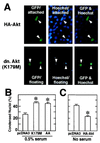



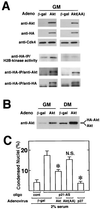

Similar articles
-
Insulin-like growth factor-mediated muscle cell survival: central roles for Akt and cyclin-dependent kinase inhibitor p21.Mol Cell Biol. 2000 Dec;20(23):8983-95. doi: 10.1128/MCB.20.23.8983-8995.2000. Mol Cell Biol. 2000. PMID: 11073997 Free PMC article.
-
AKT/PKB phosphorylation of p21Cip/WAF1 enhances protein stability of p21Cip/WAF1 and promotes cell survival.J Biol Chem. 2002 Mar 29;277(13):11352-61. doi: 10.1074/jbc.M109062200. Epub 2001 Dec 27. J Biol Chem. 2002. PMID: 11756412
-
Stem cell antigen-1 is necessary for cell-cycle withdrawal and myoblast differentiation in C2C12 cells.J Cell Sci. 2004 Dec 1;117(Pt 25):6185-95. doi: 10.1242/jcs.01548. Epub 2004 Nov 16. J Cell Sci. 2004. PMID: 15546912
-
Novel targets of Akt, p21(Cipl/WAF1), and MDM2.Semin Oncol. 2002 Jun;29(3 Suppl 11):62-70. doi: 10.1053/sonc.2002.34057. Semin Oncol. 2002. PMID: 12138399 Review.
-
Cell cycle exit upon myogenic differentiation.Curr Opin Genet Dev. 1997 Oct;7(5):597-602. doi: 10.1016/s0959-437x(97)80005-6. Curr Opin Genet Dev. 1997. PMID: 9388774 Review.
Cited by
-
MiR-696 Regulates C2C12 Cell Proliferation and Differentiation by Targeting CNTFRα.Int J Biol Sci. 2017 Mar 11;13(4):413-425. doi: 10.7150/ijbs.17508. eCollection 2017. Int J Biol Sci. 2017. PMID: 28529450 Free PMC article.
-
NOX2-derived hydrogen peroxide impedes the AMPK/Akt-mTOR signaling pathway contributing to cell death in neuronal cells.Cell Signal. 2022 Jun;94:110330. doi: 10.1016/j.cellsig.2022.110330. Epub 2022 Apr 4. Cell Signal. 2022. PMID: 35390465 Free PMC article.
-
Akt2 causes TGFβ-induced deptor downregulation facilitating mTOR to drive podocyte hypertrophy and matrix protein expression.PLoS One. 2018 Nov 16;13(11):e0207285. doi: 10.1371/journal.pone.0207285. eCollection 2018. PLoS One. 2018. PMID: 30444896 Free PMC article.
-
Runx2 induces osteoblast and chondrocyte differentiation and enhances their migration by coupling with PI3K-Akt signaling.J Cell Biol. 2004 Jul 5;166(1):85-95. doi: 10.1083/jcb.200401138. Epub 2004 Jun 28. J Cell Biol. 2004. PMID: 15226309 Free PMC article.
-
Retinoid X Receptor-selective Signaling in the Regulation of Akt/Protein Kinase B Isoform-specific Expression.J Biol Chem. 2016 Feb 5;291(6):3090-9. doi: 10.1074/jbc.M115.692707. Epub 2015 Dec 14. J Biol Chem. 2016. PMID: 26668312 Free PMC article.
References
-
- Altomare D A, Guo K, Cheng J Q, Sonoda G, Walsh K, Testa J R. Cloning, chromosomal localization and expression analysis of the mouse Akt2 oncogene. Oncogene. 1995;11:1055–1060. - PubMed
-
- Altomare D A, Lyons G E, Mitsuuchi Y, Cheng J Q, Testa J R. Akt2 mRNA is highly expressed in embryonic brown fat and AKT2 kinase is activated by insulin. Oncogene. 1998;16:2407–2411. - PubMed
-
- Bellacosa A, Franke T F, Gonzalez-Portal M E, Datta K, Taguchi T, Gardner J, Cheng J Q, Testa J R, Tsichlis P N. Structure, expression and chromosomal mapping of c-akt: relationship to v-akt and its implications. Oncogene. 1993;8:745–754. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
