Specific distribution of sodium channels in axons of rat embryo spinal motoneurones
- PMID: 10373702
- PMCID: PMC2269407
- DOI: 10.1111/j.1469-7793.1999.0203r.x
Specific distribution of sodium channels in axons of rat embryo spinal motoneurones
Abstract
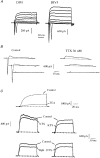
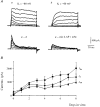
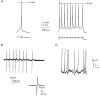
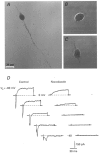
1. The distribution of Na+ channels and development of excitability were investigated in vitro in purified spinal motoneurones obtained from rat embryos at E14, using electrophysiological, immunocytochemical and autoradiographical methods. 2. One hour after plating the motoneurones (DIV0), only somas were present. They expressed a robust delayed rectifier K+ current (IDR) and a fast-inactivating A-type K+ current (IA). The rapid neuritic outgrowth was paralleled by the emergence of a fast-activating TTX-sensitive sodium current (INa), and by an increase in both K+ currents. 3. The change in the three currents was measured daily, up to DIV8. The large increase in INa observed after DIV2 was accompanied by the onset of excitability. Spontaneous activity was observed as from DIV6. 4. The occurrence of axonal differentiation was confirmed by the fact that (i) only one neurite per motoneurone generated antidromic action potentials; and (ii) 125I-alpha-scorpion toxin binding, a specific marker of Na+ channels, labelled only one neurite and the greatest density was observed in the initial segment. Na+ channels therefore selectively targeted the axon and were absent from the dendrites and somas. 5. The specific distribution of Na+ channels was detectable as soon as the neurites began to grow. When the neuritic outgrowth was blocked by nocodazole, no INa developed. 6. It was concluded that, in spinal embryonic motoneurone in cell culture, Na+ channels, the expression of which starts with neuritic differentiation, are selectively addressed to the axonal process, whereas K+ channels are present in the soma prior to the neuritic outgrowth.
Figures
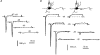

References
-
- Barry PH, Lynch JW. Liquid junction potentials and small cell effects in patch-clamp analysis. Journal of Membrane Biology. 1991;121:101–117. - PubMed
-
- Benowitz LI, Routtenberg A. GAP-43: an intrinsic determinant of neuronal development and plasticity. Trends in Neurosciences. 1997;20:84–91. - PubMed
-
- Boudier JA, Berwald-Netter Y, Dellmann HD, Boudier JL, Couraud F, Koulakoff A, Cau P. Ultrastructural visualization of Na+ channel associated 125I-α-scorpion toxin binding sites on fetal mouse nerve cells in culture. Developmental Brain Research. 1985;20:137–142. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

