Inward rectifier potassium conductance regulates membrane potential of canine colonic smooth muscle
- PMID: 10373706
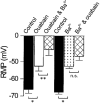
- PMCID: PMC2269411
- DOI: 10.1111/j.1469-7793.1999.0247r.x
Inward rectifier potassium conductance regulates membrane potential of canine colonic smooth muscle
Abstract
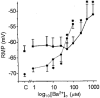
1. The membrane potential of gastrointestinal smooth muscles determines the open probability of ion channels involved in rhythmic electrical activity. The role of Ba2+-sensitive K+ conductances in the maintenance of membrane potential was examined in canine proximal colon circular muscle. 2. Application of Ba2+ (1-100 microM) to strips of tunica muscularis produced depolarization of cells along the submucosal surface of the circular muscle layer. Significantly higher concentrations of Ba2+ were needed to depolarize preparations from which the submucosal and myenteric pacemaker regions were removed. 3. Elevation of extracellular [K+]o (from 5.9 to 12 mM) brought membrane potentials closer to EK (the Nernst potential for K+ ions), suggesting activation of a K+ conductance. This occurred at potentials much more negative than the activation range for delayed rectifier channels (Kv). 4. Forskolin (1 microM) caused hyperpolarization and a leftward shift in the dose-response relationship for Ba2+, suggesting that forskolin may activate a Ba2+-sensitive conductance. 5. Patch-clamp recordings from interstitial cells of Cajal (ICC) revealed the presence of a Ba2+-sensitive inward rectifier potassium conductance. Far less of this conductance was present in smooth muscle cells. 6. Kir2.1 was expressed in the circular muscle layer of the canine proximal colon, duodenum, jejunum and ileum. Kir2.1 mRNA was expressed in greater abundance along the submucosal surface of the circular muscle layer in the colon. 7. These results demonstrate that ICC express a Ba2+-sensitive conductance (possibly encoded by Kir2.1). This conductance contributes to the generation and maintenance of negative membrane potentials between slow waves.
Figures

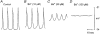





Similar articles
-
Canine colonic circular muscle generates action potentials without the pacemaker component.Can J Physiol Pharmacol. 1994 Jan;72(1):70-81. doi: 10.1139/y94-012. Can J Physiol Pharmacol. 1994. PMID: 7516818
-
Molecular and functional characterization of inwardly rectifying K+ currents in murine proximal colon.J Physiol. 2018 Feb 1;596(3):379-391. doi: 10.1113/JP275234. Epub 2017 Dec 27. J Physiol. 2018. PMID: 29205356 Free PMC article.
-
Kir2.1 encodes the inward rectifier potassium channel in rat arterial smooth muscle cells.J Physiol. 1999 Mar 15;515 ( Pt 3)(Pt 3):639-51. doi: 10.1111/j.1469-7793.1999.639ab.x. J Physiol. 1999. PMID: 10066894 Free PMC article.
-
ATP-sensitive and inwardly rectifying potassium channels in smooth muscle.Physiol Rev. 1997 Oct;77(4):1165-232. doi: 10.1152/physrev.1997.77.4.1165. Physiol Rev. 1997. PMID: 9354814 Review.
-
Potassium channels in airway smooth muscle: a tale of two channels.Pharmacol Ther. 1993;58(1):1-12. doi: 10.1016/0163-7258(93)90064-k. Pharmacol Ther. 1993. PMID: 8105495 Review.
Cited by
-
Functional expression of inward rectifier potassium channels in cultured human pulmonary smooth muscle cells: evidence for a major role of Kir2.4 subunits.J Membr Biol. 2006;213(1):19-29. doi: 10.1007/s00232-006-0037-y. Epub 2007 Mar 8. J Membr Biol. 2006. PMID: 17347781 Free PMC article.
-
Role of the hyperpolarization-activated cation current (Ih) in pacemaker activity in area postrema neurons of rat brain slices.J Physiol. 2003 Oct 1;552(Pt 1):135-48. doi: 10.1113/jphysiol.2003.047191. Epub 2003 Aug 1. J Physiol. 2003. PMID: 12897173 Free PMC article.
-
Changes in inward rectifier K+ channels in hepatic stellate cells during primary culture.Yonsei Med J. 2008 Jun 30;49(3):459-71. doi: 10.3349/ymj.2008.49.3.459. Yonsei Med J. 2008. PMID: 18581597 Free PMC article.
-
The role of K⁺ conductances in regulating membrane excitability in human gastric corpus smooth muscle.Am J Physiol Gastrointest Liver Physiol. 2015 Apr 1;308(7):G625-33. doi: 10.1152/ajpgi.00220.2014. Epub 2015 Jan 15. Am J Physiol Gastrointest Liver Physiol. 2015. PMID: 25591864 Free PMC article.
-
Characterization of the A-type potassium current in murine gastric antrum.J Physiol. 2002 Oct 15;544(2):417-28. doi: 10.1113/jphysiol.2002.025171. J Physiol. 2002. PMID: 12381815 Free PMC article.
References
-
- Barajas-Lopez C, Huizinga JD. Ouabain-induced excitation of colonic smooth muscle due to block of K+ conductance by intracellular Na+ ions. European Journal of Pharmacology. 1992;221:51–58. 10.1016/0014-2999(92)90771-U. - DOI - PubMed
-
- Berezin I, Huizinga JD, Daniel EE. Interstitial cells of Cajal in the canine colon: a special communication network at the inner border of the circular muscle. Journal of Comparative Neurology. 1988;273:42–51. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

