Interstitial cells of cajal generate electrical slow waves in the murine stomach
- PMID: 10373707
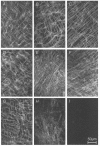
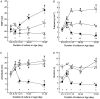
- PMCID: PMC2269418
- DOI: 10.1111/j.1469-7793.1999.0257r.x
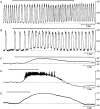
Interstitial cells of cajal generate electrical slow waves in the murine stomach
Abstract
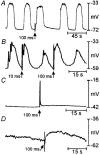
1. The gastric corpus and antrum contain interstitial cells of Cajal (ICC) within the tunica muscularis. We tested the hypothesis that ICC are involved in the generation and regeneration of electrical slow waves. 2. Normal, postnatal development of slow wave activity was characterized in tissues freshly removed from animals between birth and day 50 (D50). Slow wave amplitude and frequency increased during this period. Networks of myenteric ICC (IC-MY) were present in gastric muscles at birth and did not change significantly in appearance during the period of study as imaged by confocal immunofluorescence microscopy. 3. IC-MY networks were maintained and electrical rhythmicity developed in organ culture in a manner similar to normal postnatal development. Electrical activity was maintained for at least 48 days in culture. 4. Addition of a neutralizing antibody (ACK2) for the receptor tyrosine kinase, Kit, to the culture media caused progressive loss of Kit-immunoreactive cells. Loss of Kit-immunoreactive cells was associated with loss of slow wave activity. Most muscles became electrically quiescent after 3-4 weeks of exposure to ACK2. 5. In some muscles small clusters of Kit-immunoreactive IC-MY remained after culturing with ACK2. These muscles displayed slow wave activity but only in the immediate regions in which Kit-positive IC-MY remained. These data suggest that regions without Kit-immunoreactive cells cannot generate or regenerate slow waves. 6. After loss of Kit-immunoreactive cells, the muscles could not be paced by direct electrical stimulation. Stimulation with acetylcholine also failed to elicit slow waves. The data suggest that the generation of slow waves is an exclusive property of IC-MY; smooth muscle cells may not express the ionic apparatus necessary for generation of these events. 7. We conclude that IC-MY are an essential element in the spontaneous rhythmic electrical and contractile activity of gastric muscles. This class of ICC appears to generate slow wave activity and may provide a means for regeneration of slow waves.
Figures



Similar articles
-
Pacing of interstitial cells of Cajal in the murine gastric antrum: neurally mediated and direct stimulation.J Physiol. 2003 Dec 1;553(Pt 2):545-59. doi: 10.1113/jphysiol.2003.050419. Epub 2003 Sep 18. J Physiol. 2003. PMID: 14500772 Free PMC article.
-
Development of electrical rhythmicity in the murine gastrointestinal tract is specifically encoded in the tunica muscularis.J Physiol. 1997 Nov 15;505 ( Pt 1)(Pt 1):241-58. doi: 10.1111/j.1469-7793.1997.241bc.x. J Physiol. 1997. PMID: 9409486 Free PMC article.
-
Role of PI3-kinase in the development of interstitial cells and pacemaking in murine gastrointestinal smooth muscle.J Physiol. 1999 May 1;516 ( Pt 3)(Pt 3):835-46. doi: 10.1111/j.1469-7793.1999.0835u.x. J Physiol. 1999. PMID: 10200429 Free PMC article.
-
The interstitial cells of Cajal and a gastroenteric pacemaker system.Arch Histol Cytol. 2002 Mar;65(1):1-26. doi: 10.1679/aohc.65.1. Arch Histol Cytol. 2002. PMID: 12002607 Review.
-
Cellular mechanisms of myogenic activity in gastric smooth muscle.Jpn J Physiol. 2000 Jun;50(3):289-301. doi: 10.2170/jjphysiol.50.289. Jpn J Physiol. 2000. PMID: 11016979 Review.
Cited by
-
Voltage-dependent inward currents of interstitial cells of Cajal from murine colon and small intestine.J Physiol. 2002 Jun 15;541(Pt 3):797-810. doi: 10.1113/jphysiol.2002.018796. J Physiol. 2002. PMID: 12068041 Free PMC article.
-
Distribution of pacemaker function through the tunica muscularis of the canine gastric antrum.J Physiol. 2001 Nov 15;537(Pt 1):237-50. doi: 10.1111/j.1469-7793.2001.0237k.x. J Physiol. 2001. PMID: 11711577 Free PMC article.
-
The significance of interstitial cells in neurogastroenterology.J Neurogastroenterol Motil. 2014 Jul 31;20(3):294-317. doi: 10.5056/jnm14060. J Neurogastroenterol Motil. 2014. PMID: 24948131 Free PMC article.
-
Effects of histamine on cultured interstitial cells of cajal in murine small intestine.Korean J Physiol Pharmacol. 2013 Apr;17(2):149-56. doi: 10.4196/kjpp.2013.17.2.149. Epub 2013 Apr 10. Korean J Physiol Pharmacol. 2013. PMID: 23626477 Free PMC article.
-
Pacing of interstitial cells of Cajal in the murine gastric antrum: neurally mediated and direct stimulation.J Physiol. 2003 Dec 1;553(Pt 2):545-59. doi: 10.1113/jphysiol.2003.050419. Epub 2003 Sep 18. J Physiol. 2003. PMID: 14500772 Free PMC article.
References
-
- Berezin I, Huizinga JD, Daniel EE. Interstitial cells of Cajal in the canine colon: a special communication network at the inner border of the circular muscle. Journal of Comparative Neurology. 1988;273:42–51. - PubMed
-
- Burns AJ, Herbert TM, Ward SM, Sanders KM. Interstitial cells of Cajal in the guinea-pig gastrointestinal tract as revealed by c-Kit immunohistochemistry. Cell and Tissue Research. 1997;290:11–20. - PubMed
-
- Christensen J, Rick GA, Lowe LS. Distributions of interstitial cells of Cajal in stomach and colon of cat, dog, ferret, opossum, rat, guinea pig and rabbit. Journal of the Autonomic Nervous System. 1992;37:47–56. 10.1016/0165-1838(92)90144-6. - DOI - PubMed
-
- Connor JA, Connor C, Kreulen DL, Prosser CL, Weems WA. Pacemaker activity and propagation in intestinal smooth muscle. In: Worcel M, Vassort G, editors. Smooth Muscle Pharmacology and Physiology. Paris: INSERM; 1976. pp. 285–300.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

