Changes in excitability indices of cutaneous afferents produced by ischaemia in human subjects
- PMID: 10373711
- PMCID: PMC2269401
- DOI: 10.1111/j.1469-7793.1999.0301r.x
Changes in excitability indices of cutaneous afferents produced by ischaemia in human subjects
Abstract
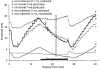
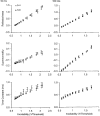
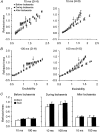
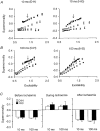
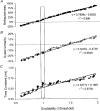
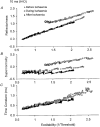
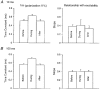
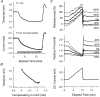
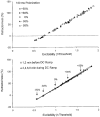
1. The present study was undertaken to determine whether mechanisms other than membrane depolarization contribute to the changes in excitability of cutaneous afferents of the median nerve under ischaemic conditions. 2. In six healthy subjects, axonal excitability was measured as the reciprocal of the threshold for a compound sensory action potential (CSAP) of 50% maximal amplitude. Refractoriness and supernormality were measured as threshold changes 2 and 7 ms, respectively, after supramaximal conditioning stimuli. The strength-duration time constant (tauSD) was calculated from the thresholds for unconditioned CSAPs using test stimuli of 0.1 and 1.0 ms duration. Changes in these indices were measured when subthreshold polarizing currents lasting 10 or 100 ms were applied, before, during and after ischaemia for 13 min. 3. At rest, the change in supernormality produced by polarizing currents was greater with the longer polarizing current, indicating that it took up to 100 ms to charge the internodal capacitance. 4. Refractoriness and its dependence on excitability increased more than expected during ischaemia. Supernormality was abolished during ischaemia, and reached a maximum after ischaemia but was then barely altered by polarizing current. tauSD had a similar relationship to excitability before, during and after ischaemia. 5. By contrast, during continuous depolarizing current for 8 min to mimic the depolarization produced by ischaemia, the relationship between excitability and refractoriness was the same during the depolarization as before it. 6. It is suggested that the large increase in refractoriness during ischaemia might be due to interference with the recovery from inactivation of transient sodium channels by an intra-axonal substrate of ischaemia. The post-ischaemic increase in supernormality and the lack of change with changes in axonal excitability can be explained by blockage of voltage-dependent potassium channels.
Figures

References
-
- Bostock H, Baker M. Evidence for two types of potassium channel in human motor axons in vivo. Brain Research. 1988;462:354–358. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

