Regulation of endothelium-derived nitric oxide production by the protein kinase Akt
- PMID: 10376602
- PMCID: PMC3637917
- DOI: 10.1038/21218
Regulation of endothelium-derived nitric oxide production by the protein kinase Akt
Erratum in
- Nature 1999 Aug 19;400(6746):792
Abstract
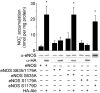
Endothelial nitric oxide synthase (eNOS) is the nitric oxide synthase isoform responsible for maintaining systemic blood pressure, vascular remodelling and angiogenesis. eNOS is phosphorylated in response to various forms of cellular stimulation, but the role of phosphorylation in the regulation of nitric oxide (NO) production and the kinase(s) responsible are not known. Here we show that the serine/threonine protein kinase Akt (protein kinase B) can directly phosphorylate eNOS on serine 1179 and activate the enzyme, leading to NO production, whereas mutant eNOS (S1179A) is resistant to phosphorylation and activation by Akt. Moreover, using adenovirus-mediated gene transfer, activated Akt increases basal NO release from endothelial cells, and activation-deficient Akt attenuates NO production stimulated by vascular endothelial growth factor. Thus, eNOS is a newly described Akt substrate linking signal transduction by Akt to the release of the gaseous second messenger NO.
Figures
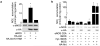


References
-
- Huang PL, et al. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377:239–242. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

