Effects of Mycoplasma fermentans incognitus on differentiation of THP-1 cells
- PMID: 10377089
- PMCID: PMC116494
- DOI: 10.1128/IAI.67.7.3188-3192.1999
Effects of Mycoplasma fermentans incognitus on differentiation of THP-1 cells
Abstract
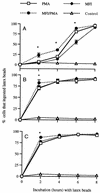
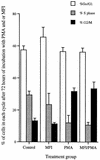
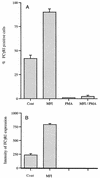
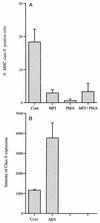
Mycoplasma fermentans incognitus has been isolated from human tissue in patients both with and without AIDS who died of systemic infection. M. fermentans incognitus and other strains of M. fermentans have been associated with rheumatoid arthritis. While cell extracts of M. fermentans incognitus can induce changes in murine and human cells of the monocytic lineage, little is known about interactions of viable organisms with such cells. Because of the central role of macrophages in chronic inflammation, we examined the effects of M. fermentans incognitus on surface markers and functions of THP-1 cells, a well-characterized human monocytic cell line. This cell line has been used extensively in studies of macrophage differentiation, especially following exposure to phorbol esters. Changes in cell morphology, phagocytosis, rate of cell division, and selected surface markers were evaluated in cultures of THP-1 cells exposed to phorbol myristate acetate (PMA), M. fermentans incognitus, or both. As reported by other investigators, PMA induced THP-1 cells to differentiate into cells resembling tissue macrophages. M. fermentans incognitus only minimally affected changes induced by PMA, slightly increasing the percentage of cells positive for FCgammaRI and major histocompatibility complex (MHC) class II antigens. M. fermentans incognitus alone induced an incomplete arrest in the cell cycle at G0 phase, increased phagocytic ability, and enhanced expression of FCgammaRI, CR3, CR4, and MHC class II antigens.
Figures

Similar articles
-
Effects of Mycoplasma fermentans on the myelomonocytic lineage. Different molecular entities with cytokine-inducing and cytocidal potential.J Immunol. 1996 Jan 15;156(2):670-8. J Immunol. 1996. PMID: 8543819
-
Evidence that Lo's mycoplasma (Mycoplasma fermentans incognitus) is not a unique strain among Mycoplasma fermentans strains.J Clin Microbiol. 1992 Sep;30(9):2435-40. doi: 10.1128/jcm.30.9.2435-2440.1992. J Clin Microbiol. 1992. PMID: 1401012 Free PMC article.
-
Characterization of MHC induction by Mycoplasma fermentans (incognitus strain).Cell Immunol. 1993 Nov;152(1):261-70. doi: 10.1006/cimm.1993.1286. Cell Immunol. 1993. PMID: 8242766
-
Models of infection due to mycoplasmas, including Mycoplasma fermentans, in the genital tract and other sites in mice.Clin Infect Dis. 1993 Aug;17 Suppl 1:S280-2. doi: 10.1093/clinids/17.supplement_1.s280. Clin Infect Dis. 1993. PMID: 8399930 Review.
-
Mycoplasma fermentans interaction with monocytes/macrophages: molecular basis.Microbes Infect. 2000 Jul;2(8):955-64. doi: 10.1016/s1286-4579(00)00395-6. Microbes Infect. 2000. PMID: 10962279 Review.
Cited by
-
Intervertebral disc cells as competent phagocytes in vitro: implications for cell death in disc degeneration.Arthritis Res Ther. 2008;10(4):R86. doi: 10.1186/ar2466. Epub 2008 Aug 1. Arthritis Res Ther. 2008. PMID: 18673547 Free PMC article.
-
Chlamydia pneumoniae infection induces differentiation of monocytes into macrophages.Infect Immun. 2002 May;70(5):2392-8. doi: 10.1128/IAI.70.5.2392-2398.2002. Infect Immun. 2002. PMID: 11953375 Free PMC article.
-
Withaferin A Associated Differential Regulation of Inflammatory Cytokines.Front Immunol. 2018 Feb 9;9:195. doi: 10.3389/fimmu.2018.00195. eCollection 2018. Front Immunol. 2018. PMID: 29479354 Free PMC article.
-
The novel angiogenic inhibitor, angiocidin, induces differentiation of monocytes to macrophages.Cancer Res. 2008 Jul 15;68(14):5905-14. doi: 10.1158/0008-5472.CAN-07-6179. Cancer Res. 2008. PMID: 18632645 Free PMC article.
-
Decades Long Involvement of THP-1 Cells as a Model for Macrophage Research: A Comprehensive Review.Antiinflamm Antiallergy Agents Med Chem. 2024;23(2):85-104. doi: 10.2174/0118715230294413240415054610. Antiinflamm Antiallergy Agents Med Chem. 2024. PMID: 38676532 Review.
References
-
- Abe T, Ohno M, Sato T, Murakami M, Kajiki M, Kodara R. “Differentiation induction” culture of human leukemic myeloid cells stimulates high production of macrophage differentiation inducing factor. Cytotechnology. 1991;5:S75–S93. - PubMed
-
- Adams D O, Hamilton T A. The cell biology of macrophage activation. Annu Rev Immunol. 1984;2:283–318. - PubMed
-
- Arnaout M A. Structure and function of the leukocyte adhesion molecules CD11/CD18. Blood. 1990;75:1037–1050. - PubMed
-
- Auwerx J. The human leukemia cell line, THP-1: a multifaceted model for the study of monocyte-macrophage differentiation. Experientia. 1991;47:22–31. - PubMed
-
- Auwerx J, Staels B, Van Vaeck F, Ceuppens J L. Changes in IgG Fc receptor expression induced by phorbol 12-myristate 13-acetate treatment of THP-1 monocyte leukemia cells. Leukoc Res. 1992;16:317–327. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

