Innate antimicrobial activity of nasal secretions
- PMID: 10377100
- PMCID: PMC116505
- DOI: 10.1128/IAI.67.7.3267-3275.1999
Innate antimicrobial activity of nasal secretions
Abstract
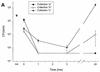
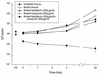
Minimally manipulated nasal secretions, an accessible form of airway surface fluid, were tested against indigenous and added bacteria by using CFU assays. Antimicrobial activity was found to vary between donors and with different target bacteria and was markedly diminished by dilution of the airway secretions. Donor-to-donor differences in electrophoresis patterns of nasal secretions in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (PAGE) and acid urea-PAGE analyses were readily observed, suggesting that polymorphic genes encode the secreted proteins. Three donors (of twenty-four total), whose nasal fluid yielded similar protein band patterns and did not kill indigenous bacteria, were determined to be heavy nasal carriers of Staphylococcus aureus. Their fluid was deficient in microbicidal activity toward a colonizing strain of S. aureus but the defect was corrected in vitro by a 1:1 addition of nasal fluid from noncarriers. The microbicidal activity of normal fluid was inactivated by heating it for 10 min to 100 degrees C and could not be restored solely by the addition of two major nasal antimicrobial proteins, lysozyme and lactoferrin. Several other known antimicrobial proteins and peptides, including statherin, secretory phospholipase A2, and defensins, were identified in nasal secretions and likely contribute to their total antimicrobial properties. Nasal fluid may serve as a useful model for the analysis of lower-airway secretions and their role in host defense against airway colonization and pulmonary infections.
Figures
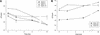



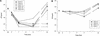
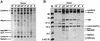
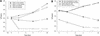
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

