Neisseria gonorrhoeae coordinately uses Pili and Opa to activate HEC-1-B cell microvilli, which causes engulfment of the gonococci
- PMID: 10377128
- PMCID: PMC116533
- DOI: 10.1128/IAI.67.7.3469-3480.1999
Neisseria gonorrhoeae coordinately uses Pili and Opa to activate HEC-1-B cell microvilli, which causes engulfment of the gonococci
Abstract
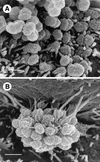
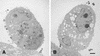
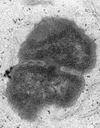
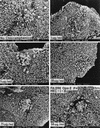
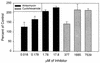
This study was undertaken to examine concomitant roles of pili and colony opacity-associated proteins (Opa) in promoting Neisseria gonorrhoeae adherence to and invasion of human endometrial HEC-1-B cells. Adherence of N. gonorrhoeae to cultured HEC-1-B cells was saturable, even though organisms adhered to <50% of the cells. During 4 to 6 h of incubation, adherent mono- and diplococci formed microcolonies on the surfaces of the cells. Microvilli of the HEC-1-B cells adhered by their distal ends to individual cocci within the microcolonies. When the microcolonies grew from isogenic pilus-negative (P-) Opa-, P- Opa+, or P+ Opa- gonococci, microvilli did not elongate, and the colonies were not engulfed. In contrast, the microvilli markedly elongated during exposure to P+ Opa+ gonococci. The microvilli adhered to the organisms along their full lengths and appeared to actively participate in the engulfment of the microcolonies. Internalized microcolonies, with P+ Opa+ gonococci, contained dividing cocci and appeared to be surrounded by cell membrane but were not clearly within vacuoles. In contrast, degenerate individual organisms were within vacuoles. Low doses of chloramphenicol, which inhibits protein synthesis by both prokaryotes and eukaryotes, prevented the microvillar response to and internalization of the P+ Opa+ gonococci; higher doses caused internalization without microvillus activation. Cycloheximide and anisomycin, which inhibit only eukaryotic protein synthesis, caused dose-dependent enhancement of uptake. Cytochalasins reduced engulfment; colchicine had no effect. These results show that gonococci must express both pili and Opa to be engulfed efficiently by HEC-1-B cells.
Figures



Similar articles
-
CEACAM is not necessary for Neisseria gonorrhoeae to adhere to and invade female genital epithelial cells.Cell Microbiol. 2001 Oct;3(10):681-91. doi: 10.1046/j.1462-5822.2001.00147.x. Cell Microbiol. 2001. PMID: 11580753
-
Effect of attachment factors (pili plus Opa) on Neisseria gonorrhoeae invasion of human fallopian tube tissue in vitro: quantitation by computerized image analysis.Microb Pathog. 1992 Aug;13(2):93-108. doi: 10.1016/0882-4010(92)90070-5. Microb Pathog. 1992. PMID: 1360614
-
Gonococcal opacity protein promotes bacterial entry-associated rearrangements of the epithelial cell actin cytoskeleton.Infect Immun. 1996 May;64(5):1621-30. doi: 10.1128/iai.64.5.1621-1630.1996. Infect Immun. 1996. PMID: 8613370 Free PMC article.
-
Adhesion of Neisseria gonorrhoeae and disease.Ciba Found Symp. 1981;80:188-201. doi: 10.1002/9780470720639.ch12. Ciba Found Symp. 1981. PMID: 6114820 Review.
-
The role of neisserial Opa proteins in interactions with host cells.Trends Microbiol. 1998 Dec;6(12):489-95. doi: 10.1016/s0966-842x(98)01365-1. Trends Microbiol. 1998. PMID: 10036728 Review.
Cited by
-
Gonococci exit apically and basally from polarized epithelial cells and exhibit dynamic changes in type IV pili.Cell Microbiol. 2006 Sep;8(9):1430-43. doi: 10.1111/j.1462-5822.2006.00722.x. Cell Microbiol. 2006. PMID: 16922862 Free PMC article.
-
Distinct Patterns of Host Adherence by Neisseria gonorrhoeae Isolated from Experimental Gonorrhea.Can J Infect Dis Med Microbiol. 2021 May 13;2021:7865405. doi: 10.1155/2021/7865405. eCollection 2021. Can J Infect Dis Med Microbiol. 2021. PMID: 34093925 Free PMC article.
-
Gonococcal invasion into epithelial cells depends on both cell polarity and ezrin.PLoS Pathog. 2021 Dec 1;17(12):e1009592. doi: 10.1371/journal.ppat.1009592. eCollection 2021 Dec. PLoS Pathog. 2021. PMID: 34852011 Free PMC article.
-
Motility and adhesion through type IV pili in Gram-positive bacteria.Biochem Soc Trans. 2016 Dec 15;44(6):1659-1666. doi: 10.1042/BST20160221. Biochem Soc Trans. 2016. PMID: 27913675 Free PMC article. Review.
-
Role of ribosomal protein L12 in gonococcal invasion of Hec1B cells.Infect Immun. 2000 Sep;68(9):5002-10. doi: 10.1128/IAI.68.9.5002-5010.2000. Infect Immun. 2000. PMID: 10948117 Free PMC article.
References
-
- Backman M, Källström H, Jonsson A-B. The phase-variable pilus-associated protein PilC is commonly expressed in clinical isolates of Neisseria gonorrhoeae, and shows sequence variability among strains. Microbiology. 1998;144:149–156. - PubMed
-
- Bhat K S, Gibbs C P, Barrera O, Morrison S G, Jähnig F, Stern A, Kupsch E M, Meyer T F, Swanson J. The opacity proteins of Neisseria gonorrhoeae strain MS11 are encoded by a family of 11 complete genes. Mol Microbiol. 1991;5:1889–1901. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

