Role of the 85-kilobase plasmid and plasmid-encoded virulence-associated protein A in intracellular survival and virulence of Rhodococcus equi
- PMID: 10377138
- PMCID: PMC116543
- DOI: 10.1128/IAI.67.7.3548-3557.1999
Role of the 85-kilobase plasmid and plasmid-encoded virulence-associated protein A in intracellular survival and virulence of Rhodococcus equi
Abstract
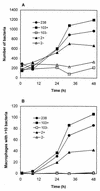
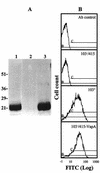
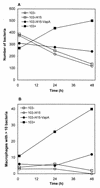
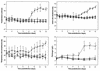
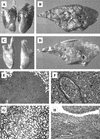
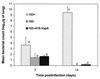
Rhodococcus equi is a facultative intracellular pathogen of macrophages and a cause of pneumonia in young horses (foals) and immunocompromised people. Isolates of R. equi from pneumonic foals typically contain large, 85- or 90-kb plasmids encoding a highly immunogenic virulence-associated protein (VapA). The objective of this study was to determine the role of the 85-kb plasmid and VapA in the intracellular survival and virulence of R. equi. Clinical isolates containing the plasmid and expressing VapA efficiently replicated within mouse macrophages in vitro, while plasmid-cured derivatives of these organisms did not multiply intracellularly. An isolate harboring the large plasmid also replicated in the tissues of experimentally infected mice, whereas its plasmid-cured derivative was rapidly cleared. All foals experimentally infected with a plasmid-containing clinical isolate developed severe bronchopneumonia, whereas the foals infected with its plasmid-cured derivative remained asymptomatic and free of visible lung lesions. By day 14 postinfection, lung bacterial burdens had increased considerably in foals challenged with the plasmid-containing clinical isolate. In contrast, bacteria could no longer be cultured from the lungs of foals challenged with the isogenic plasmid-cured derivative. A recombinant, plasmid-cured derivative expressing wild-type levels of VapA failed to replicate in macrophages and remained avirulent for both mice and foals. These results show that the 85-kb plasmid of R. equi is essential for intracellular replication within macrophages and for development of disease in the native host, the foal. However, expression of VapA alone is not sufficient to restore the virulence phenotype.
Figures

References
-
- Arlotti M, Zoboli G, Moscatelli G L, Magnati G, Maserati R, Borghi V, Andreoni M, Libanore M, Bonazzi L, Piscina A, Ciammarughi R. Rhodococcus equi infection in HIV-positive subjects: a retrospective analysis of 24 cases. Scand J Infect Dis. 1996;28:463–467. - PubMed
-
- Byrne B A, Prescott J F, Palmer G H, Hines S A. Conference handbook of the 8th International Conference on Equine Infectious Diseases, Dubai, United Arab Emirates. 1998. Characterization of a virulence-associated gene family in Rhodococcus equi, abstr. P-63; p. 236.
-
- De la Pena-Moctezuma A, Prescott J F. Association with HeLa cells by Rhodococcus equi with and without the virulence plasmid. Vet Microbiol. 1995;46:383–392. - PubMed
-
- Donisi A, Suardi M G, Casari S, Longo M, Cadeo G P, Carosi G. Rhodococcus equi infection in HIV-infected patients. AIDS. 1996;10:359–362. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

