Fatal granuloma necrosis without exacerbated mycobacterial growth in tumor necrosis factor receptor p55 gene-deficient mice intravenously infected with Mycobacterium avium
- PMID: 10377141
- PMCID: PMC116546
- DOI: 10.1128/IAI.67.7.3571-3579.1999
Fatal granuloma necrosis without exacerbated mycobacterial growth in tumor necrosis factor receptor p55 gene-deficient mice intravenously infected with Mycobacterium avium
Abstract
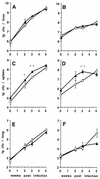
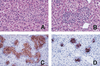
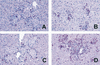
The pathogenesis of mycobacterial infections is associated with the formation of granulomas in which both antibacterial protection and tissue damage take place concomitantly. We used murine Mycobacterium avium infection to compare the development of granulomatous lesions in intravenously infected tumor necrosis factor receptor p55 (TNFRp55) gene-deficient (p55(-/-)) mice to the development of granulomatous lesions in M. avium-infected syngeneic C57BL/6 (p55(+/+)) mice. Up to 5 weeks after infection with either the highly virulent M. avium strain TMC724 or the intermediately virulent M. avium strain SE01, bacterial counts in the liver, spleen, and lung of p55(-/-) mice were identical to or lower than those in infected p55(+/+) mice. However, the formation of mononuclear cell foci in the liver was delayed by approximately 2 to 3 weeks in p55(-/-) mice compared to the results obtained for infected p55(+/+) mice. Despite comparable bacterial loads, granulomas in p55(-/-) mice underwent progressive necrosis, causing damage to the surrounding liver tissue. The appearance of necrotizing granulomas was associated with the death of all infected p55(-/-) mice, regardless of the virulence of the M. avium strain used for infection. Granulomatous lesions in the liver contained three times as many CD3(+) cells in p55(-/-) mice yet appeared more diffuse than in p55(+/+) mice. Semiquantitative reverse transcription-PCR studies revealed that prior to mouse death, interleukin-12 (IL-12) and gamma interferon mRNA levels were up regulated in the livers of infected p55(-/-) mice, while mRNA levels for tumor necrosis factor, the inducible isoform of nitric-oxide synthase (iNOS), and IL-10 were similar to those found in infected p55(+/+) mice. In response to persistent mycobacterial infection, the absence of TNFRp55 causes the disregulation of T-cell-macrophage interactions and results in fatal granuloma necrosis even when adequate antibacterial functions are maintained.
Figures



References
-
- Benini, J., E. M. Ehlers, and S. Ehlers. Different types of pulmonary grnuloma necrosis in immunocompetent vs. TNFRp55-gene-deficient mice aerogenically infected with highly virulent Mycobacterium avium. J. Pathol., in press. - PubMed
-
- Cattoretti G, Piteri S, Parraricini C, Becker M A G, Poggi S, Bifulco C, Key G, D’Amato L, Sabattini E, Fendale E, Geynolds F, Gerdes J, Rilke F. Antigen unmasking on formalin-fixed, paraffin-embedded tissue sections. J Pathol. 1993;171:83–98. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

