Susceptibility of lamivudine-resistant hepatitis B virus to other reverse transcriptase inhibitors
- PMID: 10377169
- PMCID: PMC408383
- DOI: 10.1172/JCI5882
Susceptibility of lamivudine-resistant hepatitis B virus to other reverse transcriptase inhibitors
Abstract
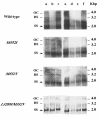
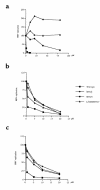
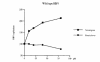
The emergence of resistant hepatitis B virus (HBV), with mutations in the YMDD motif of the polymerase gene after treatment with lamivudine, is becoming an important clinical problem. In this study, susceptibility of wild-type and lamivudine-resistant HBV M552I, M552V, and L528M/M552V mutants to other reverse transcriptase inhibitors was investigated by transient transfection of full-length HBV DNA into human hepatoma cells. HBV DNA replication was monitored by Southern blot hybridization, which showed the presence of a single-stranded band (representative of the HBV replicative intermediates) in the drug-free, wild-type HBV-transfected cells. This band was diminished in the samples of wild-type HBV DNA treated with either lamivudine, adefovir, or lobucavir. The band intensities from the lamivudine-resistant mutants were not decreased by treatment with lamivudine, but were decreased by the treatments with adefovir or lobucavir. In contrast, penciclovir and nevirapine did not diminish the intensity of the single-stranded band of wild-type HBV or the lamivudine-resistant mutants. These results demonstrate that lamivudine-resistant HBV is susceptible to adefovir and lobucavir. Lamivudine-resistant HBV should be treated with adefovir or lobucavir, and combination therapy with lamivudine and adefovir/lobucavir may prevent the emergence of lamivudine-resistant HBV.
Figures
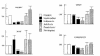

Similar articles
-
Molecular modeling and biochemical characterization reveal the mechanism of hepatitis B virus polymerase resistance to lamivudine (3TC) and emtricitabine (FTC).J Virol. 2001 May;75(10):4771-9. doi: 10.1128/JVI.75.10.4771-4779.2001. J Virol. 2001. PMID: 11312349 Free PMC article.
-
Novel nucleoside analogue MCC-478 (LY582563) is effective against wild-type or lamivudine-resistant hepatitis B virus.Antimicrob Agents Chemother. 2002 Aug;46(8):2602-5. doi: 10.1128/AAC.46.8.2602-2605.2002. Antimicrob Agents Chemother. 2002. PMID: 12121939 Free PMC article.
-
In vitro susceptibility of lamivudine-resistant hepatitis B virus to adefovir and tenofovir.Antivir Ther. 2004 Jun;9(3):353-63. Antivir Ther. 2004. PMID: 15259898
-
In vitro characterization of the anti-hepatitis B virus activity and cross-resistance profile of 2',3'-dideoxy-3'-fluoroguanosine.Antimicrob Agents Chemother. 2006 Mar;50(3):955-61. doi: 10.1128/AAC.50.3.955-961.2006. Antimicrob Agents Chemother. 2006. PMID: 16495257 Free PMC article.
-
Assessing hepatitis B virus resistance in vitro and molecular mechanisms of nucleoside resistance.Semin Liver Dis. 2002;22 Suppl 1:23-31. doi: 10.1055/s-2002-35697. Semin Liver Dis. 2002. PMID: 12447726 Review.
Cited by
-
Phenylpropenamide derivatives AT-61 and AT-130 inhibit replication of wild-type and lamivudine-resistant strains of hepatitis B virus in vitro.Antimicrob Agents Chemother. 2002 Sep;46(9):3057-60. doi: 10.1128/AAC.46.9.3057-3060.2002. Antimicrob Agents Chemother. 2002. PMID: 12183271 Free PMC article.
-
The polymerase L528M mutation cooperates with nucleotide binding-site mutations, increasing hepatitis B virus replication and drug resistance.J Clin Invest. 2001 Feb;107(4):449-55. doi: 10.1172/JCI11100. J Clin Invest. 2001. PMID: 11181644 Free PMC article.
-
Cross-resistance testing of antihepadnaviral compounds using novel recombinant baculoviruses which encode drug-resistant strains of hepatitis B virus.Antimicrob Agents Chemother. 2001 Jun;45(6):1705-13. doi: 10.1128/AAC.45.6.1705-1713.2001. Antimicrob Agents Chemother. 2001. PMID: 11353615 Free PMC article.
-
Current Options for the Therapy of Chronic Hepatitis B Infection.Curr Infect Dis Rep. 2001 Apr;3(2):137-142. doi: 10.1007/s11908-996-0036-2. Curr Infect Dis Rep. 2001. PMID: 11286654
-
Hepatitis B.Curr Treat Options Gastroenterol. 1999 Dec;2(6):463-472. doi: 10.1007/s11938-999-0050-1. Curr Treat Options Gastroenterol. 1999. PMID: 11097730
References
-
- World Health Organization warns of growing “crisis of suffering.” 1997. http://www.who.ch/whr/1997/presse.htm. - PubMed
-
- Wong DKH, et al. Effect of alpha-interferon treatment in patients with hepatitis B antigen-positive chronic hepatitis B. A meta-analysis. Ann Intern Med. 1993;119:312–323. - PubMed
-
- Summers J, Mason WS. Replication of the genome of a hepatitis B-like virus by reverse transcription of an RNA intermediate. Cell. 1982;29:403–415. - PubMed
-
- Dienstag JL, et al. A preliminary trial of lamivudine for chronic hepatitis B virus infection. N Engl J Med. 1995;333:1657–1661. - PubMed
-
- Honkoop P, de Man RA, Zondervan PE, Schalm SW. Histological improvement in patients with chronic hepatitis B virus infection treated with lamivudine. Liver. 1997;17:103–106. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

