Inhibition of TGF-beta 3 restores the invasive capability of extravillous trophoblasts in preeclamptic pregnancies
- PMID: 10377170
- PMCID: PMC408387
- DOI: 10.1172/JCI6380
Inhibition of TGF-beta 3 restores the invasive capability of extravillous trophoblasts in preeclamptic pregnancies
Abstract
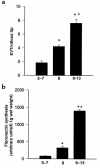
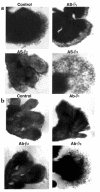
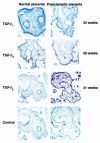
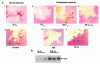
Preeclampsia, the major cause of maternal morbidity and mortality in developed countries, is associated with abnormalities of placenta function due to shallow invasion of the maternal decidua by trophoblasts. Data suggest that TGF-beta may play a role in inhibiting trophoblast outgrowth or invasion, or both. We report that placental TGF-beta 3 expression is high in early pregnancy but falls at around 9 weeks' gestation. This pattern is inversely correlated with trophoblast outgrowth and fibronectin synthesis, markers of early trophoblast differentiation toward an invasive phenotype. We demonstrate that TGF-beta 3 is overexpressed in preeclamptic placentae. In contrast to control placentae, explants from preeclamptic pregnancies fail to exhibit spontaneous invasion in vitro. Significantly, antisense-induced inhibition of TGF-beta 3 expression, and inhibition of TGF-beta 3 activity with antibodies, induces the formation of columns of trophoblast cells, which migrate out of the explant into the underlying Matrigel. To our knowledge, this is the first demonstration that the hypoinvasive placental phenotype characteristic of preeclampsia can be essentially normalized in vitro by biochemical manipulation. We speculate that a failure to downregulate expression of TGF-beta 3 at around 9 weeks' gestation results in shallow trophoblast invasion and predisposes the pregnancy to preeclampsia.
Figures

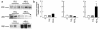
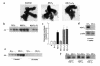

Similar articles
-
Oxygen and placental development during the first trimester: implications for the pathophysiology of pre-eclampsia.Placenta. 2000 Mar-Apr;21 Suppl A:S25-30. doi: 10.1053/plac.1999.0522. Placenta. 2000. PMID: 10831118
-
Adriana and Luisa Castellucci Award lecture 2001. Hypoxia inducible factor-1: oxygen regulation of trophoblast differentiation in normal and pre-eclamptic pregnancies--a review.Placenta. 2002 Apr;23 Suppl A:S47-57. doi: 10.1053/plac.2002.0815. Placenta. 2002. PMID: 11978059 Review.
-
Endoglin regulates trophoblast differentiation along the invasive pathway in human placental villous explants.Endocrinology. 1997 Nov;138(11):4977-88. doi: 10.1210/endo.138.11.5475. Endocrinology. 1997. PMID: 9348229
-
TGFβ signalling: a nexus between inflammation, placental health and preeclampsia throughout pregnancy.Hum Reprod Update. 2024 Jul 1;30(4):442-471. doi: 10.1093/humupd/dmae007. Hum Reprod Update. 2024. PMID: 38519450 Free PMC article. Review.
-
Dynamic changes in hyperglycosylated human chorionic gonadotrophin throughout the first trimester of pregnancy and its role in early placentation.Hum Reprod. 2015 May;30(5):1029-38. doi: 10.1093/humrep/dev016. Epub 2015 Mar 4. Hum Reprod. 2015. PMID: 25743784
Cited by
-
The Role of TGF-β during Pregnancy and Pregnancy Complications.Int J Mol Sci. 2023 Nov 28;24(23):16882. doi: 10.3390/ijms242316882. Int J Mol Sci. 2023. PMID: 38069201 Free PMC article. Review.
-
Combined Effects of Methyldopa and Baicalein or Scutellaria baicalensis Roots Extract on Blood Pressure, Heart Rate, and Expression of Inflammatory and Vascular Disease-Related Factors in Spontaneously Hypertensive Pregnant Rats.Pharmaceuticals (Basel). 2022 Oct 29;15(11):1342. doi: 10.3390/ph15111342. Pharmaceuticals (Basel). 2022. PMID: 36355514 Free PMC article.
-
Regulation of monocyte chemoattractant protein-1 expression by tumor necrosis factor-alpha and interleukin-1beta in first trimester human decidual cells: implications for preeclampsia.Am J Pathol. 2006 Feb;168(2):445-52. doi: 10.2353/ajpath.2006.050082. Am J Pathol. 2006. PMID: 16436659 Free PMC article.
-
Cytokines: Important for implantation?J Assist Reprod Genet. 2007 Nov;24(11):491-505. doi: 10.1007/s10815-007-9142-9. Epub 2007 Nov 28. J Assist Reprod Genet. 2007. PMID: 18044017 Free PMC article. Review.
-
Hypoxia and human placental development.J Clin Invest. 2000 Mar;105(5):559-60. doi: 10.1172/JCI9512. J Clin Invest. 2000. PMID: 10712424 Free PMC article. No abstract available.
References
-
- Cross JC, Werb Z, Fisher SJ. Implantation and the placenta: key pieces of the development puzzle. Science. 1994;266:1508–1518. - PubMed
-
- Strickland S, Richards WG. Invasion of trophoblasts. Cell. 1992;71:355–357. - PubMed
-
- Aplin JD. Implantation, trophoblast differentiation and haemochorial placentation: mechanistic evidence in vivo and in vitro. J Cell Sci. 1991;99:681–692. - PubMed
-
- Redman CWG. Current topic: pre-eclampsia and the placenta. Placenta. 1991;12:301–308. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

