Macrophage lipoprotein lipase promotes foam cell formation and atherosclerosis in vivo
- PMID: 10377176
- PMCID: PMC408384
- DOI: 10.1172/JCI6117
Macrophage lipoprotein lipase promotes foam cell formation and atherosclerosis in vivo
Abstract
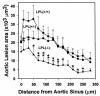
Expression of lipoprotein lipase (LPL) by the macrophage has been proposed to promote foam cell formation and atherosclerosis, primarily on the basis of in vitro studies. LPL-deficient mice might provide a model for testing the role of LPL secretion by the macrophage in an in vivo system. Unfortunately, homozygous deficiency of LPL in the mouse is lethal shortly after birth. Because the fetal liver is the major site of hematopoiesis in the developing fetus, transplantation of C57BL/6 mice with LPL-/- fetal liver cells (FLCs) was used to investigate the physiologic role of macrophage LPL expression in vivo. Thirty-four female C57BL/6 mice were lethally irradiated and reconstituted with FLCs from day 14 LPL+/+, LPL+/-, and LPL-/- donors. No significant differences were detected in plasma levels of post-heparin LPL activity or in serum cholesterol or triglyceride levels between the 3 groups on either a chow diet or an atherogenic diet. After 19 weeks on the atherogenic diet, aortae were collected for quantitative analysis of the extent of aortic atherosclerosis. LPL expression was detected by immunocytochemistry and in situ hybridization in macrophages of aortic atherosclerotic lesions of LPL+/+-->C57BL/6 and LPL+/--->C57BL/6 mice, but not in LPL-/--->C57BL/6 mice, whereas myocardial cells expressed LPL in all groups. The mean aortic lesion area was reduced by 55% in LPL-/--->C57BL/6 mice compared with LPL+/+-->C57BL/6 mice and by 45% compared with LPL+/--->C57BL/6 mice, respectively. These data demonstrate in vivo that LPL expression by macrophages in the artery wall promotes foam cell formation and atherosclerosis. off
Figures







References
-
- Brunzell, J.D. 1995. Familial lipoprotein lipase deficiency and other causes of the chylomicronemia syndrome. In The metabolic basis of inherited disease. Volume 2. C.R. Scriver, A.L. Beaudet, W.S. Sly, and D. Valle, editors. McGraw-Hill. New York, NY. 1913–1932.
-
- Saxena U, Klein MG, Goldberg IJ. Identification and characterization of the endothelial cell surface lipoprotein lipase receptor. J Biol Chem. 1991;266:17516–17521. - PubMed
-
- Zilversmit DB. Atherogenesis: a postprandial phenomenon. Circulation. 1979;60:473–485. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

