Mitogen-activated protein kinase inhibits 1,25-dihydroxyvitamin D3-dependent signal transduction by phosphorylating human retinoid X receptor alpha
- PMID: 10377179
- PMCID: PMC408392
- DOI: 10.1172/JCI6871
Mitogen-activated protein kinase inhibits 1,25-dihydroxyvitamin D3-dependent signal transduction by phosphorylating human retinoid X receptor alpha
Abstract
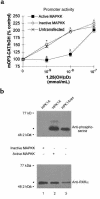
Human retinoid X receptor alpha (hRXR alpha) is a member of the nuclear receptor family of transcriptional regulators. It regulates transcription through its association with several heterodimeric partners, including the vitamin D3 receptor (VDR). Signaling through the VDR is essential for normal calcium homeostasis and has been shown to inhibit the proliferation of cancer cells derived from a number of tissues. Here we show that phosphorylation of hRXR alpha in ras-transformed human keratinocytes through the activated Ras-Raf-mitogen-activated protein kinase (Ras-Raf-MAP kinase) pathway results in attenuated transactivation by the VDR and resistance to the growth inhibitory action of 1,25 dihydroxyvitamin D3 [1,25(OH)2D3] and RXR-specific agonist LG1069 (4-[1-(5,6,7, 8-tetrahydro-3,5,5,8,8-pentamethyl-2-naphthalenyl) ethenyl]-benzoic acid). Phosphorylation of hRXR alpha occurs at serine 260, a consensus MAP kinase site. Inhibition of MAP kinase activity or point mutagenesis of serine 260 of hRXR alpha reverses the observed resistance to 1,25(OH)2D3 and LG1069. Thus, hRXR alpha is a downstream target of MAP kinase, and its phosphorylation may play an important role in malignant transformation.
Figures




References
-
- Zachos G, Spandidos GA. Expression of ras proto-oncogenes: regulation and implications in the development of human tumors. Crit Rev Oncol Hematol. 1997;26:65–75. - PubMed
-
- Davis RJ. The mitogen-activated protein kinase signal transduction pathway. J Biol Chem. 1993;268:14553–14556. - PubMed
-
- Weigel, N.L. 1994. Receptor phosphorylation in molecular biology intelligence unit. In Mechanism of steroid hormone regulation of gene transcription. M-J. Tsai, editor. R.G. Landes Co. Austin, Texas. 93–110.
-
- Haussler MR, et al. The nuclear vitamin D receptor: biological and molecular regulatory properties revealed. J Bone Miner Res. 1998;13:325–349. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

