Human herpesvirus 6 (HHV-6) causes severe thymocyte depletion in SCID-hu Thy/Liv mice
- PMID: 10377191
- PMCID: PMC2192958
- DOI: 10.1084/jem.189.12.1953
Human herpesvirus 6 (HHV-6) causes severe thymocyte depletion in SCID-hu Thy/Liv mice
Abstract
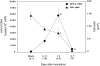
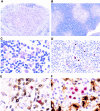
Human herpesvirus 6 (HHV-6) is a potentially immunosuppressive agent that may act as a cofactor in the progression of AIDS. Here, we describe the first small animal model of HHV-6 infection. HHV-6 subgroup A, strain GS, efficiently infected the human thymic tissue implanted in SCID-hu Thy/Liv mice, leading to the destruction of the graft. Viral DNA was detected in Thy/Liv implants by quantitative polymerase chain reaction (PCR) as early as 4 d after inoculation and peaked at day 14. The productive nature of the infection was confirmed by electron microscopy and immunohistochemical staining. Atypical thymocytes with prominent nuclear inclusions were detected by histopathology. HHV-6 replication was associated with severe, progressive thymocyte depletion involving all major cellular subsets. However, intrathymic T progenitor cells (ITTPs) appeared to be more severely depleted than the other subpopulations, and a preferred tropism of HHV-6 for ITTPs was demonstrated by quantitative PCR on purified thymocyte subsets. These findings suggest that thymocyte depletion by HHV-6 may be due to infection and destruction of these immature T cell precursors. Similar results were obtained with strain PL-1, a primary isolate belonging to subgroup B. The severity of the lesions observed in this animal model underscores the possibility that HHV-6 may indeed be immunosuppressive in humans.
Figures

Similar articles
-
Divergent effects of chronic HIV-1 infection on human thymocyte maturation in SCID-hu mice.J Immunol. 1995 Jan 15;154(2):907-21. J Immunol. 1995. PMID: 7814892
-
Induction of MHC class I expression on immature thymocytes in HIV-1-infected SCID-hu Thy/Liv mice: evidence of indirect mechanisms.J Immunol. 1999 Jun 15;162(12):7555-62. J Immunol. 1999. PMID: 10358212 Free PMC article.
-
Disseminated human immunodeficiency virus 1 (HIV-1) infection in SCID-hu mice after peripheral inoculation with HIV-1.J Exp Med. 1994 Feb 1;179(2):513-22. doi: 10.1084/jem.179.2.513. J Exp Med. 1994. PMID: 8294863 Free PMC article.
-
HIV-1 replication and pathogenesis in the human thymus.Curr HIV Res. 2003 Jul;1(3):275-85. doi: 10.2174/1570162033485258. Curr HIV Res. 2003. PMID: 15046252 Free PMC article. Review.
-
SCID-hu mice: a model for studying disseminated HIV infection.Semin Immunol. 1996 Aug;8(4):223-31. doi: 10.1006/smim.1996.0028. Semin Immunol. 1996. PMID: 8883145 Review.
Cited by
-
Induction of cell-cell fusion from without by human herpesvirus 6B.J Virol. 2006 Oct;80(19):9916-20. doi: 10.1128/JVI.02693-05. J Virol. 2006. PMID: 16973598 Free PMC article.
-
Comparative Analysis of Roseoloviruses in Humans, Pigs, Mice, and Other Species.Viruses. 2019 Nov 30;11(12):1108. doi: 10.3390/v11121108. Viruses. 2019. PMID: 31801268 Free PMC article. Review.
-
Human immunodeficiency virus type 1 pathobiology studied in humanized BALB/c-Rag2-/-gammac-/- mice.J Virol. 2007 Mar;81(6):2700-12. doi: 10.1128/JVI.02010-06. Epub 2006 Dec 20. J Virol. 2007. PMID: 17182671 Free PMC article.
-
Animal models for human herpesvirus 6 infection.Front Microbiol. 2013 Jul 4;4:174. doi: 10.3389/fmicb.2013.00174. eCollection 2013. Front Microbiol. 2013. PMID: 23847599 Free PMC article.
-
The Ban on US Government Funding Research Using Human Fetal Tissues: How Does This Fit with the NIH Mission to Advance Medical Science for the Benefit of the Citizenry?Stem Cell Reports. 2019 Nov 12;13(5):777-786. doi: 10.1016/j.stemcr.2019.10.003. Stem Cell Reports. 2019. PMID: 31722191 Free PMC article. Review.
References
-
- Salahuddin SZ, Ablashi DV, Markham PD, Josephs SF, Sturzenegger S, Kaplan M, Halligan G, Biberfeld P, Wong-Staal F, Kramarsky B, Gallo RC. Isolation of a new virus, HBLV, in patients with lymphoproliferative disorders. Science. 1986;234:596–601. - PubMed
-
- Lusso P. Human herpesvirus 6 (HHV-6) Antiviral Res. 1996;31:1–21. - PubMed
-
- Knox KK, Pietryga D, Harrington DJ, Franciosi R, Carrigan DR. Progressive immunodeficiency and fatal pneumonitis associated with human herpesvirus 6 infection in an infant. Clin Infect Dis. 1995;20:406–413. - PubMed

