The concentration of synaptically released glutamate outside of the climbing fiber-Purkinje cell synaptic cleft
- PMID: 10377338
- PMCID: PMC6782308
- DOI: 10.1523/JNEUROSCI.19-13-05265.1999
The concentration of synaptically released glutamate outside of the climbing fiber-Purkinje cell synaptic cleft
Abstract
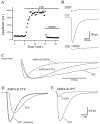
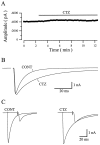
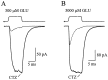
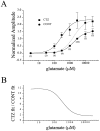
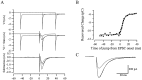
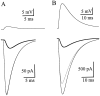
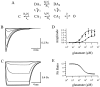
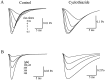
AMPA receptors and glutamate transporters expressed by cerebellar Bergmann glial cells are activated by neurotransmitter released from climbing fibers (). Based on anatomical evidence, this is most likely the result of glutamate diffusing out of the climbing fiber-Purkinje cell synaptic clefts (). We used the change in the EC50 of the Bergmann glia AMPA receptors produced by cyclothiazide (CTZ) to estimate the concentration of glutamate reached at the glial membrane. The decrease of the EC50 gives rise to a concentration-dependent potentiation of the AMPA receptor-mediated responses (). By comparing the increase in amplitude of the AMPA receptor response in the Bergmann glia (840 +/- 240%; n = 8) with the shift in the glutamate dose-response curve measured in excised patches (EC50, 1810 microM in control vs 304 microM in CTZ), we estimate that the extrasynaptic transmitter concentration reaches 160-190 microM. This contrasts with the concentration in the synaptic cleft, thought to rapidly rise above 1 mM, but is still high enough to activate glutamate receptors. These results indicate that the sphere of influence of synaptically released glutamate can extend beyond the synaptic cleft.
Figures

References
-
- Asztely F, Erdemli G, Kullmann DM. Extrasynaptic glutamate spillover in the hippocampus: dependence on temperature and the role of active glutamate uptake. Neuron. 1997;18:281–293. - PubMed
-
- Bergles DE, Jahr CE. Synaptic activation of glutamate transporters in hippocampal astrocytes. Neuron. 1997;19:1297–1308. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
