Fast excitatory synaptic transmission mediated by nicotinic acetylcholine receptors in Drosophila neurons
- PMID: 10377342
- PMCID: PMC6782340
- DOI: 10.1523/JNEUROSCI.19-13-05311.1999
Fast excitatory synaptic transmission mediated by nicotinic acetylcholine receptors in Drosophila neurons
Abstract
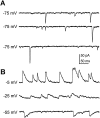
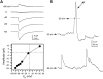
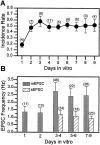
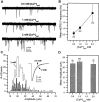
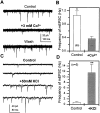
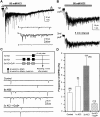
Difficulty in recording from single neurons in vivo has precluded functional analyses of transmission at central synapses in Drosophila, where the neurotransmitters and receptors mediating fast synaptic transmission have yet to be identified. Here we demonstrate that spontaneously active synaptic connections form between cultured neurons prepared from wild-type embryos and provide the first direct evidence that both acetylcholine and GABA mediate fast interneuronal synaptic transmission in Drosophila. The predominant type of fast excitatory transmission between cultured neurons is mediated by nicotinic acetylcholine receptors (nAChRs). Detailed analysis of cholinergic transmission reveals that spontaneous EPSCs (sEPSCs) are composed of both evoked and action potential-independent [miniature EPSC (mEPSC)] components. The mEPSCs are characterized by a broad, positively skewed amplitude histogram in which the variance is likely to reflect differences in the currents induced by single quanta. Biophysical characteristics of the cholinergic mEPSCs include a rapid rise time (0.6 msec) and decay (tau = 2 msec). Regulation of mEPSC frequency by external calcium and cobalt suggests that calcium influx through voltage-gated channels influences the probability of ACh release. In addition, brief depolarization of the cultures with KCl can induce a calcium-dependent increase in sEPSC frequency that persists for up to 3 hr after termination of the stimulus, illustrating one form of plasticity at these cholinergic synapses. These data demonstrate that cultured embryonic neurons, amenable to both genetic and biochemical manipulations, present a unique opportunity to define genes/signal transduction cascades involved in functional regulation of fast excitatory transmission at interneuronal cholinergic synapses in Drosophila.
Figures





References
-
- Banker G, Goslin K. Culturing nerve cells. Cellular and molecular neuroscience series. MIT; Cambridge, MA: 1991.
-
- Bekkers JM, Stevens CF. NMDA and non-NMDA receptors are co-localized at individual excitatory synapses in cultured rat hippocampus. Nature. 1989;341:230–233. - PubMed
-
- Bertrand D, Ballivet M, Gomez M, Bertrand S, Phannavong B, Gundelfinger ED. Physiological properties of neuronal nicotinic receptors reconstituted from the vertebrate β2 subunit and Drosophila α-subunits. Eur J Neurosci. 1994;6:869–875. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
