The native rat olfactory cyclic nucleotide-gated channel is composed of three distinct subunits
- PMID: 10377344
- PMCID: PMC6782342
- DOI: 10.1523/JNEUROSCI.19-13-05332.1999
The native rat olfactory cyclic nucleotide-gated channel is composed of three distinct subunits
Abstract
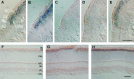
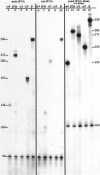
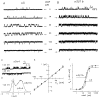
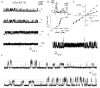
Cyclic nucleotide-gated (CNG) channels play central roles in visual and olfactory signal transduction. In the retina, rod photoreceptors express the subunits CNCalpha1 and CNCbeta1a. In cone photoreceptors, only CNCalpha2 expression has been demonstrated so far. Rat olfactory sensory neurons (OSNs) express two homologous subunits, here designated CNCalpha3 and CNCalpha4. This paper describes the characterization of CNCbeta1b, a third subunit expressed in OSNs and establishes it as a component of the native channel. CNCbeta1b is an alternate splice form of the rod photoreceptor CNCbeta1a subunit. Analysis of mRNA and protein expression together suggest co-expression of all three subunits in sensory cilia of OSNs. From single-channel analyses of native rat olfactory channels and of channels expressed heterologously from all possible combinations of the CNCalpha3, -alpha4, and -beta1b subunits, we conclude that the native CNG channel in OSNs is composed of all three subunits. Thus, CNG channels in both rod photoreceptors and olfactory sensory neurons result from coassembly of specific alpha subunits with various forms of an alternatively spliced beta subunit.
Figures





References
-
- Ardell MD, Aragon I, Oliveira L, Porche GE, Burke E, Pittler SJ. The β subunit of human rod photoreceptor cGMP-gated cation channel is generated from a complex transcription unit. FEBS Lett. 1996;389:213–218. - PubMed
-
- Bakalyar HA, Reed RR. Identification of a specialized adenylyl cyclase that may mediate odorant detection. Science. 1990;250:1403–1406. - PubMed
-
- Balasubramanian S, Lynch JW, Barry PH. Calcium-dependent modulation of the agonist affinity of the mammalian olfactory cyclic nucleotide-gated channel by calmodulin and a novel endogenous factor. J Membr Biol. 1996;152:13–23. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
