Optic flow input to the hippocampal formation from the accessory optic system
- PMID: 10377360
- PMCID: PMC6782317
- DOI: 10.1523/JNEUROSCI.19-13-05514.1999
Optic flow input to the hippocampal formation from the accessory optic system
Abstract
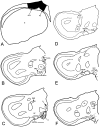
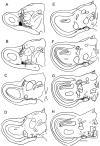
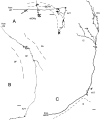
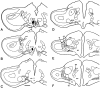
Recent studies in rodents have implicated the hippocampal formation in "path integration": the ability to use self-motion cues (ideothesis) to guide spatial behavior. Such models of hippocampal function assume that self-motion information arises from the vestibular system. In the present study we used the retrograde tracer cholera toxin subunit B, the anterograde tracer biotinylated dextran amine, and standard extracellular recording techniques to investigate whether the hippocampal formation [which consists of the hippocampus proper and the area parahippocampalis (Hp/APH) in pigeons] receives information from the accessory optic system (AOS). The AOS is a visual pathway dedicated to the analysis of the "optic flow fields" that result from self-motion. Optic flow constitutes a rich source of ideothetic information that could be used for navigation. Both the nucleus of the basal optic root (nBOR) and nucleus lentiformis mesencephali of the AOS were shown to project to the area ventralis of Tsai (AVT), which in turn was shown to project to the Hp/APH. A smaller direct projection from the nBOR pars dorsalis to the hippocampus was also revealed. During extracellular recording experiments, about half of the cells within the AVT responded to optic flow stimuli. Together these results illustrate that the Hp/APH receives information about self-motion from the AOS. We postulate that this optic flow information is used for path integration. A review of the current literature suggests that an analogous neuronal circuit exists in mammals, but it has simply been overlooked.
Figures


Similar articles
-
Telencephalic projections to the nucleus of the basal optic root and pretectal nucleus lentiformis mesencephali in pigeons.Vis Neurosci. 2005 Mar-Apr;22(2):237-47. doi: 10.1017/S0952523805221090. Vis Neurosci. 2005. PMID: 15935115
-
Projections from the accessory optic system and pretectum to the dorsolateral thalamus in the pigeon (Columbia livia): a study using both anteretrograde and retrograde tracers.J Comp Neurol. 1998 Feb 22;391(4):456-69. J Comp Neurol. 1998. PMID: 9486825
-
Retinal projection to the pretectal nucleus lentiformis mesencephali in pigeons (Columba livia).J Comp Neurol. 2014 Dec 1;522(17):3928-42. doi: 10.1002/cne.23649. Epub 2014 Aug 13. J Comp Neurol. 2014. PMID: 25044056
-
Spatial separation of visual and vestibular processing in the human hippocampal formation.Ann N Y Acad Sci. 2011 Sep;1233:177-86. doi: 10.1111/j.1749-6632.2011.06115.x. Ann N Y Acad Sci. 2011. PMID: 21950991 Review.
-
Anatomy of the avian hippocampal formation.Rev Neurosci. 2006;17(1-2):3-15. doi: 10.1515/revneuro.2006.17.1-2.3. Rev Neurosci. 2006. PMID: 16703939 Review.
Cited by
-
Spatial response properties of homing pigeon hippocampal neurons: correlations with goal locations, movement between goals, and environmental context in a radial-arm arena.J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2004 Dec;190(12):1047-62. doi: 10.1007/s00359-004-0562-z. Epub 2004 Sep 23. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2004. PMID: 15449093
-
Long-term effects of permanent vestibular lesions on hippocampal spatial firing.J Neurosci. 2003 Jul 23;23(16):6490-8. doi: 10.1523/JNEUROSCI.23-16-06490.2003. J Neurosci. 2003. PMID: 12878690 Free PMC article.
-
Construction and disruption of spatial memory networks during development.Learn Mem. 2019 Jun 17;26(7):206-218. doi: 10.1101/lm.049239.118. Print 2019 Jul. Learn Mem. 2019. PMID: 31209115 Free PMC article. Review.
-
How the cerebellum may monitor sensory information for spatial representation.Front Syst Neurosci. 2014 Nov 4;8:205. doi: 10.3389/fnsys.2014.00205. eCollection 2014. Front Syst Neurosci. 2014. PMID: 25408638 Free PMC article. Review.
-
Spatial cognition and the avian hippocampus: Research in domestic chicks.Front Psychol. 2022 Sep 23;13:1005726. doi: 10.3389/fpsyg.2022.1005726. eCollection 2022. Front Psychol. 2022. PMID: 36211859 Free PMC article. Review.
References
-
- Arends JJA, Zeigler HP. Organization of the cerebellum in the pigeon (Columba livia): II. Projections of the cerebellar nuclei. J Comp Neurol. 1991;306:245–272. - PubMed
-
- Bingman VP, Yates G. Hippocampal lesions impair navigational learning in experienced homing pigeons. Behav Neurosci. 1992;106:229–232. - PubMed
-
- Bingman VP, Ioale P, Casini G, Bagnoli P. The avian hippocampus: evidence for a role in the development of the homing pigeon navigational map. Behav Neurosci. 1990;104:906–911. - PubMed
-
- Blair HT, Sharp PE. Visual and vestibular influences on head-direction cells in the anterior thalamus of the rat. Behav Neurosci. 1996;110:643–660. - PubMed
-
- Brecha N, Karten HJ, Hunt SP. Projections of the nucleus of basal optic root in the pigeon: an autoradiographic and horseradish peroxidase study. J Comp Neurol. 1980;189:615–670. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
