Spontaneous activity of neostriatal cholinergic interneurons in vitro
- PMID: 10377365
- PMCID: PMC6782311
- DOI: 10.1523/JNEUROSCI.19-13-05586.1999
Spontaneous activity of neostriatal cholinergic interneurons in vitro
Abstract
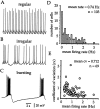
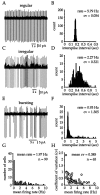
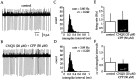
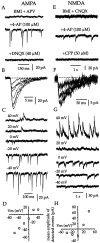
Neostriatal cholinergic interneurons fire irregularly but tonically in vivo. The summation of relatively few depolarizing potentials and their temporal sequence are thought to underlie spike triggering and the irregularity of action potential timing, respectively. In these experiments we used whole-cell, cell-attached, and extracellular recording techniques to investigate the role of spontaneous synaptic inputs in the generation and patterning of action potentials in cholinergic interneurons in vitro. Cholinergic cells were spontaneously active in vitro at 25 +/- 1 degrees C during whole-cell recording from 2 to 3 week postnatal slices and at 35 +/- 2 degrees C during cell-attached and extracellular recording from 3 to 4 week postnatal slices. A range of firing frequencies and patterns was observed including regular, irregular, and burst firing. Blockade of AMPA and NMDA receptors altered neither the firing rate nor the pattern, and accordingly, voltage-clamp data revealed a very low incidence of spontaneous EPSCs. GABAA receptor antagonists were also ineffective in altering the spiking frequency or pattern owing to minimal inhibitory input in vitro. Functional excitatory and inhibitory inputs to cholinergic cells were disclosed after application of 4-aminopyridine (100 microM), indicating that these synapses are present but not active in vitro. Blockade of D1 or D2 dopamine receptors or muscarinic receptors also failed to influence tonic activity in cholinergic cells. Together these data indicate that cholinergic interneurons are endogenously active and generate action potentials in the absence of any synaptic input. Interspike interval histograms and autocorrelograms generated from unit recordings of cholinergic cells in vitro were indistinguishable from those of tonically active neurons recorded in vivo. Irregular spiking is therefore embedded in the mechanism responsible for endogenous activity.
Figures



References
-
- Agard DA, Hiraoka Y, Shaw P, Sedat JW. Fluorescence microscopy in three dimensions. Methods Cell Biol. 1989;30:353–377. - PubMed
-
- Aghajanian GK, Rasmussen K. Intracellular studies in the facial nucleus illustrating a simple new method for obtaining viable motoneurons in adult rat brain slices. Synapse. 1989;3:331–338. - PubMed
-
- Akins PT, Surmeier DJ, Kitai ST. Muscarinic modulation of a transient K+ conductance in rat neostriatal neurons. Nature. 1990;344:240–242. - PubMed
-
- Aosaki T, Graybiel AM, Kimura M. Effect of the nigrostriatal dopamine system on acquired neural responses in the striatum of behaving monkeys. Science. 1994a;265:412–415. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
