Binocular neurons in V1 of awake monkeys are selective for absolute, not relative, disparity
- PMID: 10377367
- PMCID: PMC6782336
- DOI: 10.1523/JNEUROSCI.19-13-05602.1999
Binocular neurons in V1 of awake monkeys are selective for absolute, not relative, disparity
Abstract
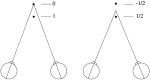
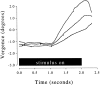
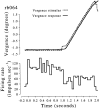
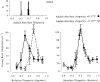
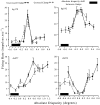
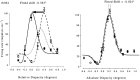
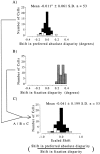
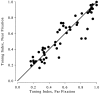
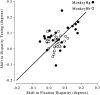
Most neurophysiological accounts of disparity selectivity in neurons of the primary visual cortex (V1) imply that they are selective for absolute retinal disparities. By contrast, a number of psychophysical observations indicate that relative disparities play a more important role in depth perception. During recordings from disparity selective neurons in area V1 of awake behaving monkeys, we used a disparity feedback loop () to add controlled amounts of absolute disparity to a display containing both absolute and relative disparities. This manipulation changed the absolute disparity of all the visible features in the display but left unchanged the relative disparities signalled by these features. The addition of absolute disparities produced clear changes in the neural responses to unchanged external stimuli, which were well predicted by the measured change in absolute disparity: in 45/53 cases, the neuron maintained a consistent firing pattern with respect to absolute disparity so that the manipulation created no significant change in the absolute disparity preferred by the neuron. No neuron in V1 maintained a consistent relationship with relative disparity. We conclude that the relative disparity signals used in primate depth perception are constructed outside area V1.
Figures







References
-
- Bishop PO, Henry GH. Spatial vision. Annu Rev Psychol. 1971;22:119–160. - PubMed
-
- Brookes A, Stevens KA. The analogy between stereo depth and brightness. Perception. 1989;18:601–614. - PubMed
-
- Cumming BG, Judge SJ. Disparity-induced and blur-induced convergence eye movement and accommodation in the monkey. J Neurophysiol. 1986;55:896–914. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
