Heat-inactivated proteins are rescued by the DnaK.J-GrpE set and ClpB chaperones
- PMID: 10377389
- PMCID: PMC22047
- DOI: 10.1073/pnas.96.13.7184
Heat-inactivated proteins are rescued by the DnaK.J-GrpE set and ClpB chaperones
Abstract
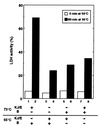
Functional chaperone cooperation between Hsp70 (DnaK) and Hsp104 (ClpB) was demonstrated in vitro. In a eubacterium Thermus thermophilus, DnaK and DnaJ exist as a stable trigonal ring complex (TDnaK.J complex) and the dnaK gene cluster contains a clpB gene. When substrate proteins were heated at high temperature, none of the chaperones protected them from heat inactivation, but the TDnaK.J complex could suppress the aggregation of proteins in an ATP- and TGrpE-dependent manner. Subsequent incubation of these heated preparations at moderate temperature after addition of TClpB resulted in the efficient reactivation of the proteins. Reactivation was also observed, even though the yield was low, if the substrate protein alone was heated and incubated at moderate temperature with the TDnaK.J complex, TGrpE, TClpB, and ATP. Thus, all these components were necessary for the reactivation. Further, we found that TGroEL/ES could not substitute TClpB.
Figures

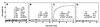

References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

