Ezrin, a plasma membrane-microfilament linker, signals cell survival through the phosphatidylinositol 3-kinase/Akt pathway
- PMID: 10377409
- PMCID: PMC22080
- DOI: 10.1073/pnas.96.13.7300
Ezrin, a plasma membrane-microfilament linker, signals cell survival through the phosphatidylinositol 3-kinase/Akt pathway
Abstract
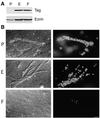
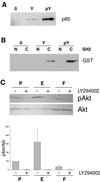
ERM (Ezrin-Radixin-Moesin) proteins function as plasma membrane-actin cytoskeleton linkers and participate in the formation of specialized domains of the plasma membrane. We have investigated ezrin function in tubulogenesis of a kidney-derived epithelial cell line, LLC-PK1. Here we show that cells overproducing a mutant form of ezrin in which Tyr-353 was changed to a phenylalanine (Y353F) undergo apoptosis when assayed for tubulogenesis. While investigating the mechanism responsible for this apoptosis, we found that ezrin interacts with p85, the regulatory subunit of phosphatidylinositol 3-kinase (PI 3-kinase). Two distinct sites of ezrin are involved in this interaction, the amino-terminal domain containing the first 309 aa and the phosphorylated Tyr-353 residue, which binds to the carboxyl-terminal SH2 domain of p85. Cells producing Y353F ezrin are defective in activation of the protein kinase Akt, a downstream target of PI 3-kinase that protects cells against apoptosis. Furthermore, the apoptotic phenotype of these cells is rescued by production of a constitutively activated form of PI 3-kinase. Taken together, these results establish a novel function for ezrin in determining survival of epithelial cells by activating the PI 3-kinase/Akt pathway.
Figures


References
-
- Bretscher A, Reczek D, Berryman M. J Cell Sci. 1997;110:3011–3018. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

