The intracellular parasite Theileria parva protects infected T cells from apoptosis
- PMID: 10377411
- PMCID: PMC22082
- DOI: 10.1073/pnas.96.13.7312
The intracellular parasite Theileria parva protects infected T cells from apoptosis
Abstract
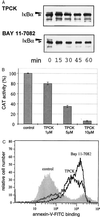
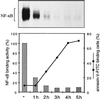
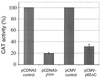
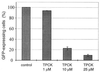
Parasites have evolved a plethora of strategies to ensure their survival. The intracellular parasite Theileria parva secures its propagation and spreads through the infected animal by infecting and transforming T cells, inducing their continuous proliferation and rendering them metastatic. In previous work, we have shown that the parasite induces constitutive activation of the transcription factor NF-kappaB, by inducing the constitutive degradation of its cytoplasmic inhibitors. The biological significance of NF-kappaB activation in T. parva-infected cells, however, has not yet been defined. Cells that have been transformed by viruses or oncogenes can persist only if they manage to avoid destruction by the apoptotic mechanisms that are activated on transformation and that contribute to maintain cellular homeostasis. We now demonstrate that parasite-induced NF-kappaB activation plays a crucial role in the survival of T. parva-transformed T cells by conveying protection against an apoptotic signal that accompanies parasite-mediated transformation. Consequently, inhibition of NF-kappaB nuclear translocation and the expression of dominant negative mutant forms of components of the NF-kappaB activation pathway, such as IkappaBalpha or p65, prompt rapid apoptosis of T. parva-transformed T cells. Our findings offer important insights into parasite survival strategies and demonstrate that parasite-induced constitutive NF-kappaB activation is an essential step in maintaining the transformed phenotype of the infected cells.
Figures
Similar articles
-
Interference by the intracellular parasite Theileria parva with T-cell signal transduction pathways induces transformation and protection against apoptosis.Vet Immunol Immunopathol. 1999 Dec 15;72(1-2):95-100. doi: 10.1016/s0165-2427(99)00121-x. Vet Immunol Immunopathol. 1999. PMID: 10614498 Review.
-
Parasite-mediated nuclear factor kappaB regulation in lymphoproliferation caused by Theileria parva infection.Proc Natl Acad Sci U S A. 1997 Nov 11;94(23):12527-32. doi: 10.1073/pnas.94.23.12527. Proc Natl Acad Sci U S A. 1997. PMID: 9356483 Free PMC article.
-
Theileria parva: taking control of host cell proliferation and survival mechanisms.Cell Microbiol. 2000 Apr;2(2):91-9. doi: 10.1046/j.1462-5822.2000.00045.x. Cell Microbiol. 2000. PMID: 11207566 Review.
-
The Akt/PKB pathway is constitutively activated in Theileria-transformed leucocytes, but does not directly control constitutive NF-kappaB activation.Cell Microbiol. 2001 Aug;3(8):537-50. doi: 10.1046/j.1462-5822.2001.00134.x. Cell Microbiol. 2001. PMID: 11488815
-
A schizont-derived protein, TpSCOP, is involved in the activation of NF-kappaB in Theileria parva-infected lymphocytes.Mol Biochem Parasitol. 2010 Nov;174(1):8-17. doi: 10.1016/j.molbiopara.2010.06.005. Epub 2010 Jun 9. Mol Biochem Parasitol. 2010. PMID: 20540970
Cited by
-
Pathogens hijack the epigenome: a new twist on host-pathogen interactions.Am J Pathol. 2014 Apr;184(4):897-911. doi: 10.1016/j.ajpath.2013.12.022. Epub 2014 Feb 11. Am J Pathol. 2014. PMID: 24525150 Free PMC article. Review.
-
Eimeria tenella ROP kinase EtROP1 induces G0/G1 cell cycle arrest and inhibits host cell apoptosis.Cell Microbiol. 2019 Jul;21(7):e13027. doi: 10.1111/cmi.13027. Epub 2019 Apr 24. Cell Microbiol. 2019. PMID: 30941872 Free PMC article.
-
Dual RNA-seq to catalogue host and parasite gene expression changes associated with virulence of T. annulata-transformed bovine leukocytes: towards identification of attenuation biomarkers.Sci Rep. 2023 Oct 24;13(1):18202. doi: 10.1038/s41598-023-45458-9. Sci Rep. 2023. PMID: 37875584 Free PMC article.
-
Comparative Pathobiology of the Intestinal Protozoan Parasites Giardia lamblia, Entamoeba histolytica, and Cryptosporidium parvum.Pathogens. 2019 Jul 29;8(3):116. doi: 10.3390/pathogens8030116. Pathogens. 2019. PMID: 31362451 Free PMC article. Review.
-
Survival of protozoan intracellular parasites in host cells.EMBO Rep. 2004 Dec;5(12):1142-7. doi: 10.1038/sj.embor.7400299. EMBO Rep. 2004. PMID: 15577928 Free PMC article. Review.
References
-
- Morrison W I, Lalor P A, Goddeeris B M, Teale A J. In: Parasite Antigens, Toward New Strategies for Vaccines. Pearson T W, editor. New York: Dekker; 1986. pp. 167–213.
-
- Brown C G, Stagg D A, Purnell R E, Kanhai G K, Payne R C. Nature (London) 1973;245:101–103. - PubMed
-
- Baldwin C L, Teale A J. Eur J Immunol. 1987;17:1859–1862. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

