Branching morphogenesis independent of mesenchymal-epithelial contact in the developing kidney
- PMID: 10377414
- PMCID: PMC22085
- DOI: 10.1073/pnas.96.13.7330
Branching morphogenesis independent of mesenchymal-epithelial contact in the developing kidney
Abstract
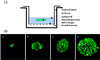
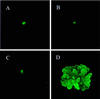
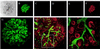
Whether mesenchymal-epithelial interactions leading to branching morphogenesis in developing epithelial tissues such as the kidney require direct cell-cell contact or are due to soluble mediators elaborated by the inducing tissue has been the subject of much debate. Here we demonstrate that ureteric bud (UB) epithelium, from which the kidney collecting system and upper urinary tract are derived, can undergo impressive three-dimensional branching morphogenesis when cultured in the appropriate extracellular matrix context in the absence of direct contact with mesenchymal tissue, indicating that the program for branching morphogenesis is inherent to the UB. Both a soluble factor in BSN cell-conditioned medium (BSN-CM) derived from an immortalized cell line thought to originate in the early metanephric mesenchyme and glial cell line-derived neurotrophic factor (GDNF) were required for early and later events in branching morphogenesis. In the absence of BSN-CM, the isolated UB did not survive; a similar result was obtained in the presence of neutralizing antibodies against glial cell line-derived neurotrophic factor. Preliminary analysis of key activity present in BSN-CM indicates that it is a heat-sensitive, heparin-binding factor with a probable molecular mass greater than 100 kDa. When the in vitro cultured UB was recombined with freshly isolated metanephric mesenchyme, nephric units were induced in the mesenchyme, and the UB branches underwent elongation. Our data suggest that, although UB branching morphogenesis per se does not require direct mesenchymal contact, such contact may play a key role in regulating branch elongation and establishing the pattern of branching. The results also suggest an approach to in vitro engineering of nephron.
Figures



Similar articles
-
Multiple fibroblast growth factors support growth of the ureteric bud but have different effects on branching morphogenesis.Mech Dev. 2001 Dec;109(2):123-35. doi: 10.1016/s0925-4773(01)00592-5. Mech Dev. 2001. PMID: 11731227
-
Identification of pleiotrophin as a mesenchymal factor involved in ureteric bud branching morphogenesis.Development. 2001 Sep;128(17):3283-93. doi: 10.1242/dev.128.17.3283. Development. 2001. PMID: 11546745
-
Matrix metalloproteinases and their inhibitors regulate in vitro ureteric bud branching morphogenesis.Am J Physiol Renal Physiol. 2000 Nov;279(5):F891-900. doi: 10.1152/ajprenal.2000.279.5.F891. Am J Physiol Renal Physiol. 2000. PMID: 11053050
-
Relevance of extracellular matrix, its receptors, and cell adhesion molecules in mammalian nephrogenesis.Am J Physiol. 1998 Oct;275(4):F467-77. doi: 10.1152/ajprenal.1998.275.4.F467. Am J Physiol. 1998. PMID: 9755118 Review.
-
Molecular mechanism of ureteric bud development.Semin Cell Dev Biol. 2003 Aug;14(4):217-24. doi: 10.1016/s1084-9521(03)00024-7. Semin Cell Dev Biol. 2003. PMID: 14627120 Review.
Cited by
-
Autocrine inhibition of cell motility can drive epithelial branching morphogenesis in the absence of growth.Philos Trans R Soc Lond B Biol Sci. 2020 Sep 14;375(1807):20190386. doi: 10.1098/rstb.2019.0386. Epub 2020 Jul 27. Philos Trans R Soc Lond B Biol Sci. 2020. PMID: 32713299 Free PMC article.
-
The contribution of branching morphogenesis to kidney development and disease.Nat Rev Nephrol. 2016 Dec;12(12):754-767. doi: 10.1038/nrneph.2016.157. Epub 2016 Nov 7. Nat Rev Nephrol. 2016. PMID: 27818506 Review.
-
Concise review: can the intrinsic power of branching morphogenesis be used for engineering epithelial tissues and organs?Stem Cells Transl Med. 2013 Dec;2(12):993-1000. doi: 10.5966/sctm.2013-0076. Epub 2013 Nov 4. Stem Cells Transl Med. 2013. PMID: 24191267 Free PMC article. Review.
-
Constructing kidney-like tissues from cells based on programs for organ development: toward a method of in vitro tissue engineering of the kidney.Tissue Eng Part A. 2010 Aug;16(8):2441-55. doi: 10.1089/ten.TEA.2009.0548. Tissue Eng Part A. 2010. PMID: 20214453 Free PMC article.
-
Hs2st mediated kidney mesenchyme induction regulates early ureteric bud branching.Dev Biol. 2010 Mar 15;339(2):354-65. doi: 10.1016/j.ydbio.2009.12.033. Epub 2010 Jan 6. Dev Biol. 2010. PMID: 20059993 Free PMC article.
References
-
- Grobstein C. Science. 1953;118:52–55. - PubMed
-
- Grobstein C. Nature (London) 1953;172:869–871. - PubMed
-
- Grobstein C. J Exp Zool. 1955;130:319–339.
-
- Saxen L. Organogenesis of the Kidney. Cambridge: Cambridge Univ. Press; 1987.
-
- Nigam S K, Aperia A, Brenner B M. In: The Kidney. Brenner B M, Rector F C, editors. Philadelphia: Saunders; 1995. pp. 72–98.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

