Conditional requirement for the Flk-1 receptor in the in vitro generation of early hematopoietic cells
- PMID: 10377421
- PMCID: PMC22092
- DOI: 10.1073/pnas.96.13.7370
Conditional requirement for the Flk-1 receptor in the in vitro generation of early hematopoietic cells
Abstract
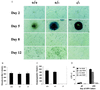
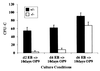
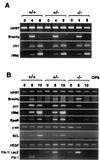
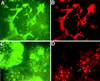
Genetic studies in mice have previously demonstrated an intrinsic requirement for the vascular endothelial growth factor (VEGF) receptor Flk-1 in the early development of both the hematopoietic and endothelial cell lineages. In this study, embryonic stem (ES) cells homozygous for a targeted null mutation in flk-1 (flk-1 (-/-)) were examined for their hematopoietic potential in vitro during embryoid body (EB) formation or when cultured on the stromal cell line OP9. Surprisingly, in EB cultures flk-1 (-/-) ES cells were able to differentiate into all myeloid-erythroid lineages, albeit at half the frequency of heterozygous lines. In contrast, although flk-1 (-/-) ES cells formed mesodermal-like colonies on OP9 monolayers, they failed to generate hematopoietic clusters even in the presence of exogenous cytokines. However, flk-1 (-/-) OP9 cultures did contain myeloid precursors, albeit at greatly reduced percentages. This defect was rescued by first allowing flk-1 (-/-) ES cells to differentiate into EBs and then passaging these cells onto OP9 stroma. Thus, the requirement for Flk-1 in early hematopoietic development can be abrogated by alterations in the microenvironment. This finding is consistent with a role for Flk-1 in regulating the migration of early mesodermally derived precursors into a microenvironment that is permissive for hematopoiesis.
Figures

Similar articles
-
In vitro hematopoietic and endothelial potential of flk-1(-/-) embryonic stem cells and embryos.Proc Natl Acad Sci U S A. 1999 Mar 2;96(5):2159-64. doi: 10.1073/pnas.96.5.2159. Proc Natl Acad Sci U S A. 1999. PMID: 10051611 Free PMC article.
-
Formation of transformed endothelial cells in the absence of VEGFR-2/Flk-1 by Polyoma middle T oncogene.Oncogene. 1999 Jul 22;18(29):4200-10. doi: 10.1038/sj.onc.1203014. Oncogene. 1999. PMID: 10435633
-
KDR/Flk-1 is a major regulator of vascular endothelial growth factor-induced tumor development and angiogenesis in murine hepatocellular carcinoma cells.Hepatology. 1999 Nov;30(5):1179-86. doi: 10.1002/hep.510300509. Hepatology. 1999. PMID: 10534339
-
[Receptors and development of endothelial and hematopoietic cells].J Soc Biol. 1999;193(2):155-7. J Soc Biol. 1999. PMID: 10451349 Review. French.
-
Segregation of the embryonic vascular and hemopoietic systems.Biochem Cell Biol. 1998;76(6):939-46. Biochem Cell Biol. 1998. PMID: 10392707 Review.
Cited by
-
Multilineage embryonic hematopoiesis requires hypoxic ARNT activity.Genes Dev. 1999 Oct 1;13(19):2478-83. doi: 10.1101/gad.13.19.2478. Genes Dev. 1999. PMID: 10521392 Free PMC article.
-
Combinatorial effects of Flk1 and Tal1 on vascular and hematopoietic development in the mouse.Genes Dev. 2003 Feb 1;17(3):380-93. doi: 10.1101/gad.1049803. Genes Dev. 2003. PMID: 12569129 Free PMC article.
-
Prenatal transplantation of cytokine-stimulated marrow improves early chimerism in a resistant strain combination but results in poor long-term engraftment.Exp Hematol. 2006 Sep;34(9):1278-87. doi: 10.1016/j.exphem.2006.05.007. Exp Hematol. 2006. PMID: 16939821 Free PMC article.
-
Molecular and developmental biology of the hemangioblast.Dev Dyn. 2008 May;237(5):1218-31. doi: 10.1002/dvdy.21542. Dev Dyn. 2008. PMID: 18429046 Free PMC article. Review.
-
Hematopoietic differentiation of human embryonic stem cells progresses through sequential hematoendothelial, primitive, and definitive stages resembling human yolk sac development.Blood. 2005 Aug 1;106(3):860-70. doi: 10.1182/blood-2004-11-4522. Epub 2005 Apr 14. Blood. 2005. PMID: 15831705 Free PMC article.
References
-
- Medvinsky A L, Samoylina N L, Muller A M, Dzierzak E A. Nature (London) 1993;364:64–67. - PubMed
-
- Godin I E, Garcia-Porrero J A, Coutinho A, Dieterlen-Lievre F, Marcos M A R. Nature (London) 1993;364:67–70. - PubMed
-
- Morrison-Graham K, Schatteman G C, Bork T, Bowen-Pope D F, Weston J A. Development (Cambridge, UK) 1992;115:133–142. - PubMed
-
- Orr-Urtreger A, Bedford M T, Do M S, Eisenbach L, Lonai P. Development (Cambridge, UK) 1992;115:289–303. - PubMed
-
- Bernstein A, Forrester L, Reith A D, Dubreuil P, Rottapel R. Semin Hematol. 1991;28:138–142. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

