Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1
- PMID: 10377430
- PMCID: PMC22101
- DOI: 10.1073/pnas.96.13.7421
Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1
Abstract
Although genetic analysis has demonstrated that members of the winged helix, or forkhead, family of transcription factors play pivotal roles in the regulation of cellular differentiation and proliferation, both during development and in the adult, little is known of the mechanisms underlying their regulation. Here we show that the activation of phosphatidylinositol 3 (PI3) kinase by extracellular growth factors induces phosphorylation, nuclear export, and transcriptional inactivation of FKHR1, a member of the FKHR subclass of the forkhead family of transcription factors. Protein kinase B (PKB)/Akt, a key mediator of PI3 kinase signal transduction, phosphorylated recombinant FKHR1 in vitro at threonine-24 and serine-253. Mutants FKHR1(T24A), FKHR1(S253A), and FKHR1(T24A/S253A) were resistant to both PKB/Akt-mediated phosphorylation and PI3 kinase-stimulated nuclear export. These results indicate that phosphorylation by PKB/Akt negatively regulates FKHR1 by promoting export from the nucleus.
Figures
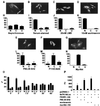

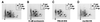

References
-
- Kaufmann E, Knöchel W. Mech Dev. 1996;57:3–20. - PubMed
-
- Hromas R, Costa R. Crit Rev Oncol Hematol. 1995;20:129–140. - PubMed
-
- Vogt P K, Li J, Freyaldenhoven B S. Virology. 1997;238:1–7. - PubMed
-
- Barr F G. Curr Top Microbiol Immunol. 1997;220:113–129. - PubMed
-
- Hillion J, Le Coniat M, Jonveaux P, Berger R, Bernard O A. Blood. 1997;90:3714–3719. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

