X inactivation and somatic cell selection rescue female mice carrying a Piga-null mutation
- PMID: 10377440
- PMCID: PMC22111
- DOI: 10.1073/pnas.96.13.7479
X inactivation and somatic cell selection rescue female mice carrying a Piga-null mutation
Abstract
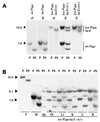
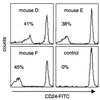
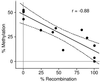
A somatic mutation in the X linked PIGA gene is responsible for the deficiency of glycosyl phosphatidylinositol (GPI)-anchored proteins on blood cells from patients with paroxysmal nocturnal hemoglobinuria. No inherited form of GPI-anchor deficiency has been described. Because conventional Piga gene knockout is associated with high embryonic lethality in chimeric mice, we used the Cre/loxP system. We generated mice in which two loxP sites flank part of Piga exon 2. After crossbreeding with female mice of the EIIa-cre strain, the floxed allele undergoes Cre-mediated recombination with high efficiency during early embryonic development. Because of X chromosome inactivation, female offspring are mosaic for cells that express or lack GPI-linked proteins. Analysis of mosaic mice showed that in heart, lung, kidney, brain, and liver, mainly wild-type Piga is active, suggesting that these tissues require GPI-linked proteins. The salient exceptions were spleen, thymus, and red blood cells, which had almost equal numbers of cells expressing the wild-type or the recombined allele, implying that GPI-linked proteins are not essential for the derivation of these tissues. PIGA(-) cells had no growth advantage, suggesting that other factors are needed for their clonal dominance in patients with paroxysmal nocturnal hemoglobinuria.
Figures


Similar articles
-
FES-Cre targets phosphatidylinositol glycan class A (PIGA) inactivation to hematopoietic stem cells in the bone marrow.J Exp Med. 2001 Sep 3;194(5):581-9. doi: 10.1084/jem.194.5.581. J Exp Med. 2001. PMID: 11535627 Free PMC article.
-
GATA1-Cre mediates Piga gene inactivation in the erythroid/megakaryocytic lineage and leads to circulating red cells with a partial deficiency in glycosyl phosphatidylinositol-linked proteins (paroxysmal nocturnal hemoglobinuria type II cells).Blood. 2001 Oct 1;98(7):2248-55. doi: 10.1182/blood.v98.7.2248. Blood. 2001. PMID: 11568013
-
Increased sensitivity to complement and a decreased red blood cell life span in mice mosaic for a nonfunctional Piga gene.Blood. 1999 Nov 1;94(9):2945-54. Blood. 1999. PMID: 10556176
-
Glycosyl phosphatidylinositol (GPI)-anchored molecules and the pathogenesis of paroxysmal nocturnal hemoglobinuria.Crit Rev Oncol Hematol. 2000 Jan;33(1):25-43. doi: 10.1016/s1040-8428(99)00052-9. Crit Rev Oncol Hematol. 2000. PMID: 10714960 Review.
-
Paroxysmal nocturnal hemoglobinuria: insights from recent advances in molecular biology.Transfus Med Rev. 2001 Oct;15(4):255-67. doi: 10.1053/tmrv.2001.26958. Transfus Med Rev. 2001. PMID: 11668433 Review.
Cited by
-
Cre-mediated germline mosaicism: a new transgenic mouse for the selective removal of residual markers from tri-lox conditional alleles.Nucleic Acids Res. 2003 Mar 1;31(5):e21. doi: 10.1093/nar/gng021. Nucleic Acids Res. 2003. PMID: 12595570 Free PMC article.
-
FES-Cre targets phosphatidylinositol glycan class A (PIGA) inactivation to hematopoietic stem cells in the bone marrow.J Exp Med. 2001 Sep 3;194(5):581-9. doi: 10.1084/jem.194.5.581. J Exp Med. 2001. PMID: 11535627 Free PMC article.
-
Analyzing clinical and genetic characteristics of a cohort with multiple congenital anomalies-hypotonia-seizures syndrome (MCAHS).Orphanet J Rare Dis. 2020 Mar 27;15(1):78. doi: 10.1186/s13023-020-01365-0. Orphanet J Rare Dis. 2020. PMID: 32220244 Free PMC article.
-
Molecular basis of cleft palates in mice.World J Biol Chem. 2015 Aug 26;6(3):121-38. doi: 10.4331/wjbc.v6.i3.121. World J Biol Chem. 2015. PMID: 26322171 Free PMC article. Review.
-
Multiple Congenital Anomalies-Hypotonia-Seizures Syndrome 2 Caused by a Novel PIGA Variant Not Associated with a Skewed X-Inactivation Pattern.Genes (Basel). 2024 Jun 18;15(6):802. doi: 10.3390/genes15060802. Genes (Basel). 2024. PMID: 38927738 Free PMC article.
References
-
- Miyata T, Takeda J, Iida Y, Yamada N, Inoue N, Takahashi M, Maeda K, Kitani T, Kinoshita T. Science. 1993;259:1318–1320. - PubMed
-
- Takeda J, Miyata T, Kawagoe K, Iida Y, Endo Y, Fujita T, Takahashi M, Kitani T, Kinoshita T. Cell. 1993;73:703–711. - PubMed
-
- Bessler M, Hillmen P, Longo L, Luzzatto L, Mason P J. Hum Mol Genet. 1994;3:751–757. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

