Identification of a metaplastic cell lineage associated with human gastric adenocarcinoma
- PMID: 10378506
- PMCID: PMC12398956
Identification of a metaplastic cell lineage associated with human gastric adenocarcinoma
Abstract
Metaplastic cell lineages arising in response to chronic injury are precursors for the evolution of dysplasia and adenocarcinoma. Although a subtype of intestinal metaplasia has been associated with gastric adenocarcinoma, the link between this lineage and the evolution of gastric adenocarcinoma has remained unclear. Wang et al (1998) have reported that an aberrant metaplastic cell lineage with morphological characteristics similar to Brunner's glands of the duodenum develops in the fundic mucosa of mice infected with Helicobacter felis. This metaplastic lineage expresses the trefoil peptide spasmolytic polypeptide (SP). Given the epidemiological association of Helicobacter species infection with gastric cancer, we hypothesized that this SP-expressing metaplastic (SPEM) lineage may represent a precursor to or appear commensurate with gastric adenocarcinoma. The SPEM lineage was present in 68% of fundic biopsies from patients with fundic Helicobacterpylori-associated gastritis, but was absent in biopsies of fundic mucosa from patients without H. pylori infection. In a review of archival samples from 22 resected gastric adenocarcinomas, we found the SPEM lineage in 91% of cases, typically located in mucosa adjacent to the carcinoma or areas of dysplasia. Importantly, 59% of resections showed SP immunoreactivity within dysplastic cells. These data indicate a strong association of the SPEM lineage with both chronic H. pylori infection and gastric adenocarcinoma.
Figures
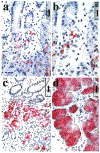

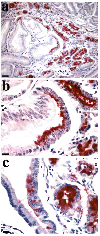
References
-
- Ahnen DJ, Poulsom R, Stamp GWH, Elia G, Pike C, Jeffery R, Longcroft J, Rio M, Chambon P, and Wright NA (1994). The ulceration-associated cell lineage (UACL) reiterates the Brunner’s gland differentiation programme but acquires the proliferative organization of the gastric gland. J Pathol 173:317–326. - PubMed
-
- Alison MR, Chinery R, Poulsom R, Ashwood P, Longcroft JM, and Wright NA (1995). Experimental ulceration leads to sequential expression of spasmolytic polypeptide, intestinal trefoil factor, epidermal growth factor and transforming growth factor alpha mRNAs in rat stomach. J Pathol 175:405–414. - PubMed
-
- Babyatsky MW, DeBeaumont M, Thim L, and Podolsky DK (1996). Oral trefoil peptides protect against ethanol- and indomethacin-induced gastric injury in rats. Gastroenterology 110:489–497. - PubMed
-
- Beck S, Schmitt H, Shizuya H, Blin N, and Gott P (1996). Cloning of contiguous genomic fragments from human chromosome 21 harbouring three trefoil peptide genes. Hum Genet 98:233–235. - PubMed
-
- Bluth RF, Carpenter HA, Pittelkow MR, Page DL, and Coffey RJ (1995). Immunolocalization of transforming growth factor-alpha in normal and diseased human gastric mucosa. Hum Pathol 26:1333–1340. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
